Consider a block of ice, with a mass of 2.00 kg and an initial temperature of -8.00°C. Given the following constants below, how much heat (in kJ) must be added to completely transform the block of ice at -8.00°C to ice at the melting point of water? *
Consider a block of ice, with a mass of 2.00 kg and an initial temperature of -8.00°C. Given the following constants below, how much heat (in kJ) must be added to completely transform the block of ice at -8.00°C to ice at the melting point of water? *
College Physics
11th Edition
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Raymond A. Serway, Chris Vuille
Chapter11: Energy In Thermal Processes
Section: Chapter Questions
Problem 3CQ: Substance A has twice the specific heat of substance B. Equal masses of the two substances, at...
Related questions
Question
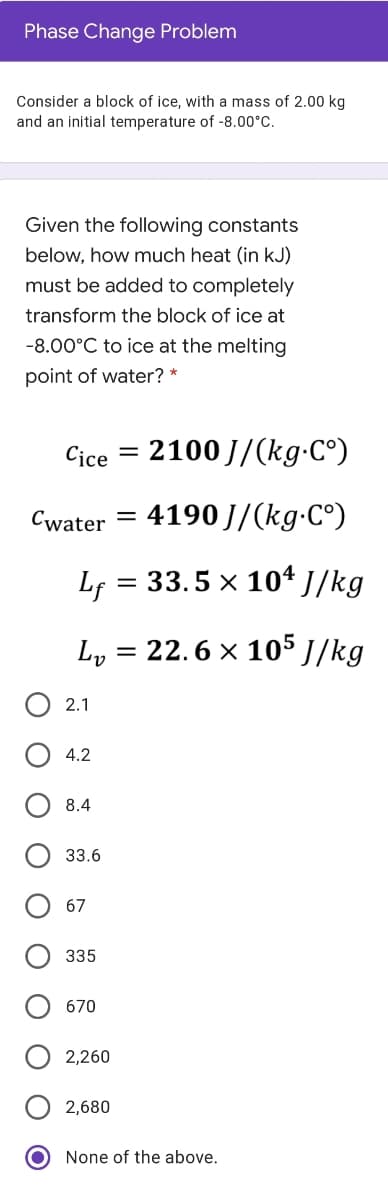
Transcribed Image Text:Phase Change Problem
Consider a block of ice, with a mass of 2.00 kg
and an initial temperature of -8.00°C.
Given the following constants
below, how much heat (in kJ)
must be added to completely
transform the block of ice at
-8.00°C to ice at the melting
point of water? *
Cice
2100 J/(kg-C°)
Cwater
4190 J/(kg-C°)
Lf = 33.5 x 10ª J/kg
Ly
22.6 x 105 J/kg
О 2.1
4.2
8.4
33.6
67
335
670
2,260
2,680
None of the above.
O O
O O
O O O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College


College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College


Glencoe Physics: Principles and Problems, Student…
Physics
ISBN:
9780078807213
Author:
Paul W. Zitzewitz
Publisher:
Glencoe/McGraw-Hill

An Introduction to Physical Science
Physics
ISBN:
9781305079137
Author:
James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:
Cengage Learning
