Chemical Principles in the Laboratory
11th Edition
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Chapter17: Classification Of Chemical Substances
Section: Chapter Questions
Problem 1ASA
Related questions
Question
Consider a d8 compound [like Pt(II)]. What is more likely to form a 4-coordinate or a 6-coordinate compound.
Explain using VB theory and CFT.
Good hand written explanation
Asap
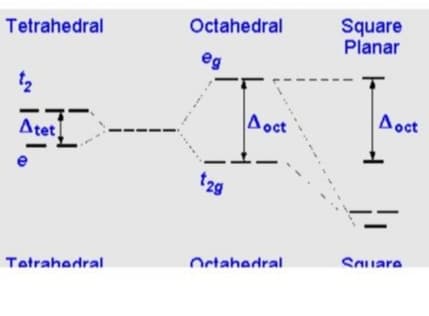
Transcribed Image Text:Tetrahedral
ܕܐ
Atet
Tetrahedral
Octahedral
eg
Aoct
12g
Octahedral
Square
Planar
A oct
Square
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole


Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole
