Consider a N₂ molecule in its first excited electronic state, that is, when an electron in the highest occupied molecular orbital (HOMO) is promoted to the lowest unoccupied molecular orbital (LUMO). Identify the molecular orbital involved and sketch a diagram to show the transition. Compare the bond order and bond length of N₂* with N₂, where the asterisk denotes the excited molecule. Is N₂* diamagnetic or paramagnetic?
Consider a N₂ molecule in its first excited electronic state, that is, when an electron in the highest occupied molecular orbital (HOMO) is promoted to the lowest unoccupied molecular orbital (LUMO). Identify the molecular orbital involved and sketch a diagram to show the transition. Compare the bond order and bond length of N₂* with N₂, where the asterisk denotes the excited molecule. Is N₂* diamagnetic or paramagnetic?
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter10: Molecular Structure And Bonding Theories
Section: Chapter Questions
Problem 10.101QE: The molecular orbital diagram of NO shown in Figure 10.47 also applies to OF. Draw the complete...
Related questions
Question
q3
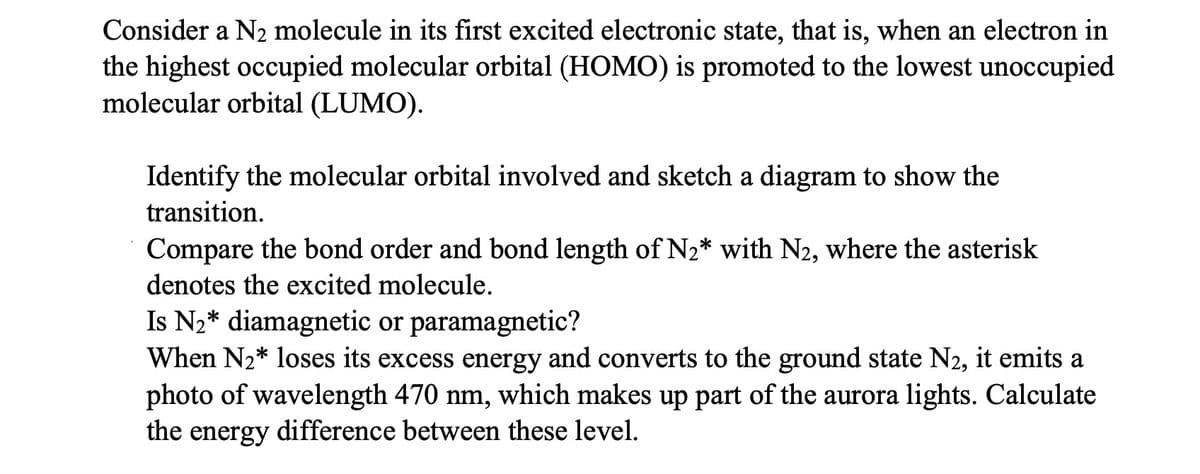
Transcribed Image Text:Consider a N2 molecule in its first excited electronic state, that is, when an electron in
the highest occupied molecular orbital (HOMO) is promoted to the lowest unoccupied
molecular orbital (LUMO).
Identify the molecular orbital involved and sketch a diagram to show the
transition.
Compare the bond order and bond length of N₂* with N2, where the asterisk
denotes the excited molecule.
Is N₂* diamagnetic or paramagnetic?
When N₂* loses its excess energy and converts to the ground state N2, it emits a
photo of wavelength 470 nm, which makes up part of the aurora lights. Calculate
the energy difference between these level.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
d

Transcribed Image Text:Consider a N2 molecule in its first excited electronic state, that is, when an electron in
the highest occupied molecular orbital (HOMO) is promoted to the lowest unoccupied
molecular orbital (LUMO).
(d) When N₂* loses its excess energy and converts to the ground state N₂, it emits a
photo of wavelength 470 nm, which makes up part of the aurora lights. Calculate
the energy difference between these level.
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning