Consider an ideal gas enclosed in a 1.00 L container at an internal pressure of 18.0 atm. Calculate the work, w, if the gas expands against a constant external pressure of 1.00 atm to a final volume of 18.0 L. J Now calculate the work done if this process is carried out in two steps. 1. First, let the gas expand against a constant external pressure of 1.80 atm to a volume of 10.0 L. 2. From the end point of step 1, let the gas expand to 18.0 L against a constant external pressure of 1.00 atm. J * TOOLS x10
Consider an ideal gas enclosed in a 1.00 L container at an internal pressure of 18.0 atm. Calculate the work, w, if the gas expands against a constant external pressure of 1.00 atm to a final volume of 18.0 L. J Now calculate the work done if this process is carried out in two steps. 1. First, let the gas expand against a constant external pressure of 1.80 atm to a volume of 10.0 L. 2. From the end point of step 1, let the gas expand to 18.0 L against a constant external pressure of 1.00 atm. J * TOOLS x10
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter17: Chemcial Thermodynamics
Section: Chapter Questions
Problem 17.33QE: A 220-L cylinder contains an ideal gas at a pressure of 150 atm. If the gas is allowed to expand...
Related questions
Question
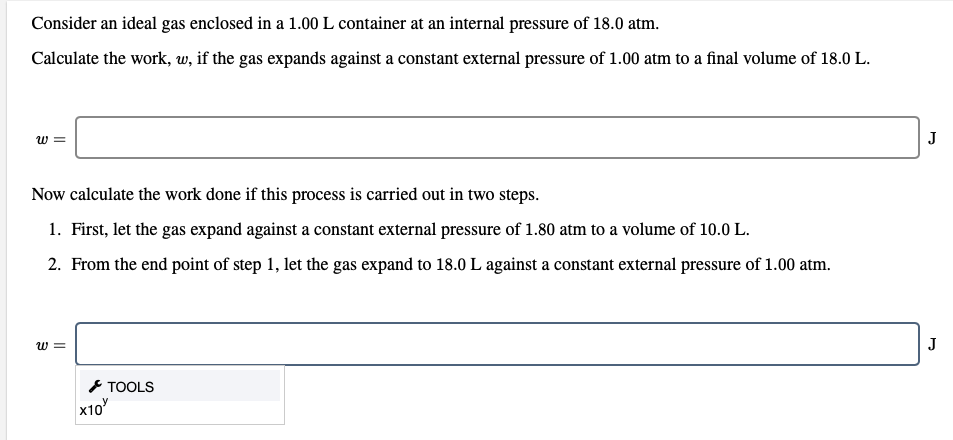
Transcribed Image Text:Consider an ideal gas enclosed in a 1.00 L container at an internal pressure of 18.0 atm.
Calculate the work, w, if the gas expands against a constant external pressure of 1.00 atm to a final volume of 18.0 L.
J
Now calculate the work done if this process is carried out in two steps.
1. First, let the gas expand against a constant external pressure of 1.80 atm to a volume of 10.0 L.
2. From the end point of step 1, let the gas expand to 18.0 L against a constant external pressure of 1.00 atm.
J
* TOOLS
x10
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning