Consider the bilowin 500 K. PCI5 (g) PCI3 (g) + Cl2 (g) When 0.24 moles of PCI5 (g) are added to the equilibrium system at constant temperatur The value of K. The value of Qc The reaction must O run in the forward direction to restablish equilibrium. O run in the reverse direction to restablish equilibrium. O remain the same. It is already at equilibrium.
Consider the bilowin 500 K. PCI5 (g) PCI3 (g) + Cl2 (g) When 0.24 moles of PCI5 (g) are added to the equilibrium system at constant temperatur The value of K. The value of Qc The reaction must O run in the forward direction to restablish equilibrium. O run in the reverse direction to restablish equilibrium. O remain the same. It is already at equilibrium.
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter12: Chemical Equilibrium
Section: Chapter Questions
Problem 12.98PAE
Related questions
Question
For the first one the options are increases, decreases, and remains the same.
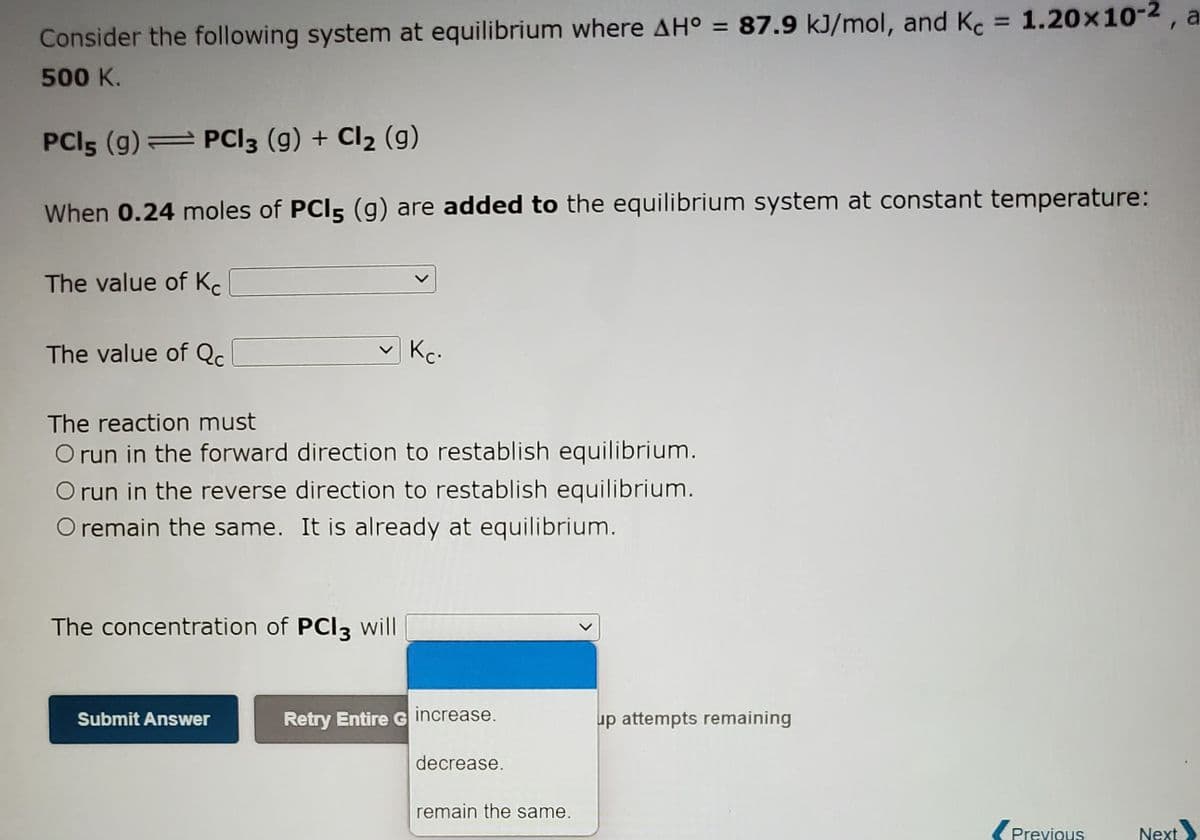
Transcribed Image Text:%3D
Consider the following system at equilibrium where AH° = 87.9 kJ/mol, and Kc 1.20x10-2, a
500 K.
PCI5 (g) PCI3 (g) + Cl2 (g)
When 0.24 moles of PCI5 (g) are added to the equilibrium system at constant temperature:
The value of K.
The value of Qc.
Kc.
The reaction must
O run in the forward direction to restablish equilibrium.
O run in the reverse direction to restablish equilibrium.
O remain the same. It is already at equilibrium.
The concentration of PCI3 will
Retry Entire G increase.
up attempts remaining
Submit Answer
decrease.
remain the same.
Previous
Next
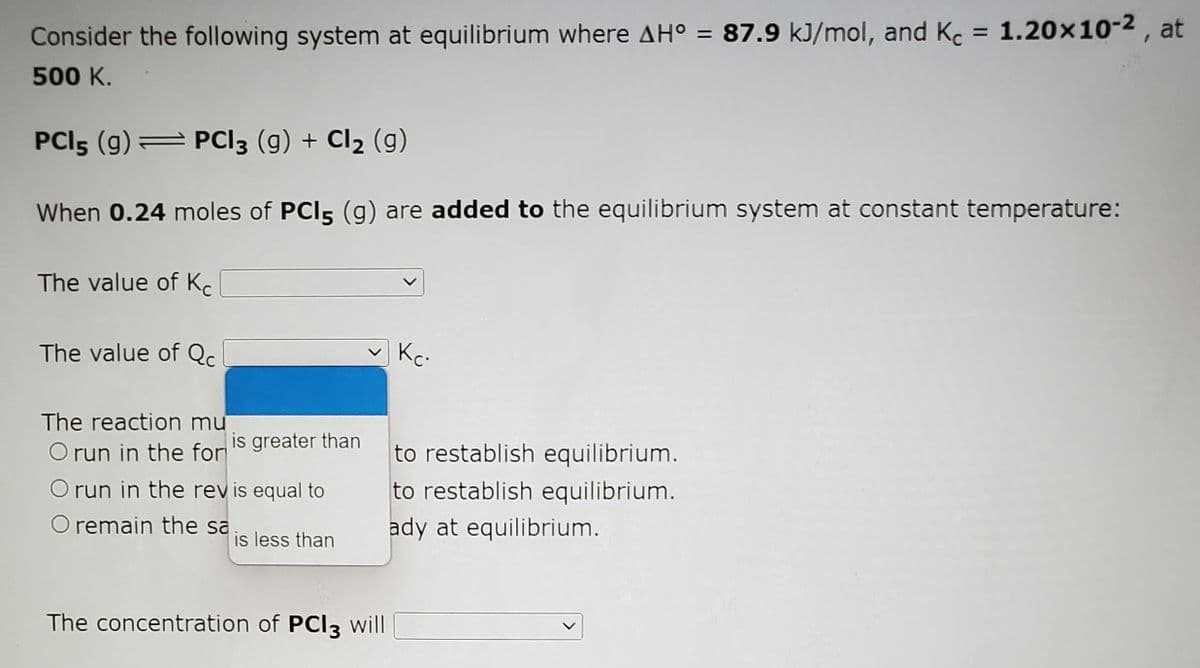
Transcribed Image Text:Consider the following system at equilibrium where AH° = 87.9 kJ/mol, and Kc :
c = 1.20x10-2 , at
%3D
500 K.
PCI5 (g) PCI3 (g) + Cl2 (g)
When 0.24 moles of PCI5 (g) are added to the equilibrium system at constant temperature:
The value of K.
The value of Qc
Kc-
The reaction mu
O run in the for
is greater than
to restablish equilibrium.
O run in the rev is equal to
to restablish equilibrium.
O remain the sa
ady at equilibrium.
is less than
The concentration of PCI3 will
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning