Consider the equilibrium system described by the chemical reaction below, which has a value of Kc equal to 1.2 x 10 at a certain temperature. If a solid sample of NH.SH decomposes, what will the equilibrium concentration of NH: be? NH:SH(s) = NHs(g) + H:S(g) 1 3 NEXT Based on the given values, set up ICE table in order to determine the unknown. NH:SH(s) NH:(g) H:S(g) Initial (M) Change (M) Eguilibrium (M)
Consider the equilibrium system described by the chemical reaction below, which has a value of Kc equal to 1.2 x 10 at a certain temperature. If a solid sample of NH.SH decomposes, what will the equilibrium concentration of NH: be? NH:SH(s) = NHs(g) + H:S(g) 1 3 NEXT Based on the given values, set up ICE table in order to determine the unknown. NH:SH(s) NH:(g) H:S(g) Initial (M) Change (M) Eguilibrium (M)
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter13: Fundamental Equilibrium Concepts
Section: Chapter Questions
Problem 95E: Consider the equilibrium 4NO2(g)+6H2O(g)4NH3(g)+7O2(g) (a) What is the expression for the...
Related questions
Question
100%
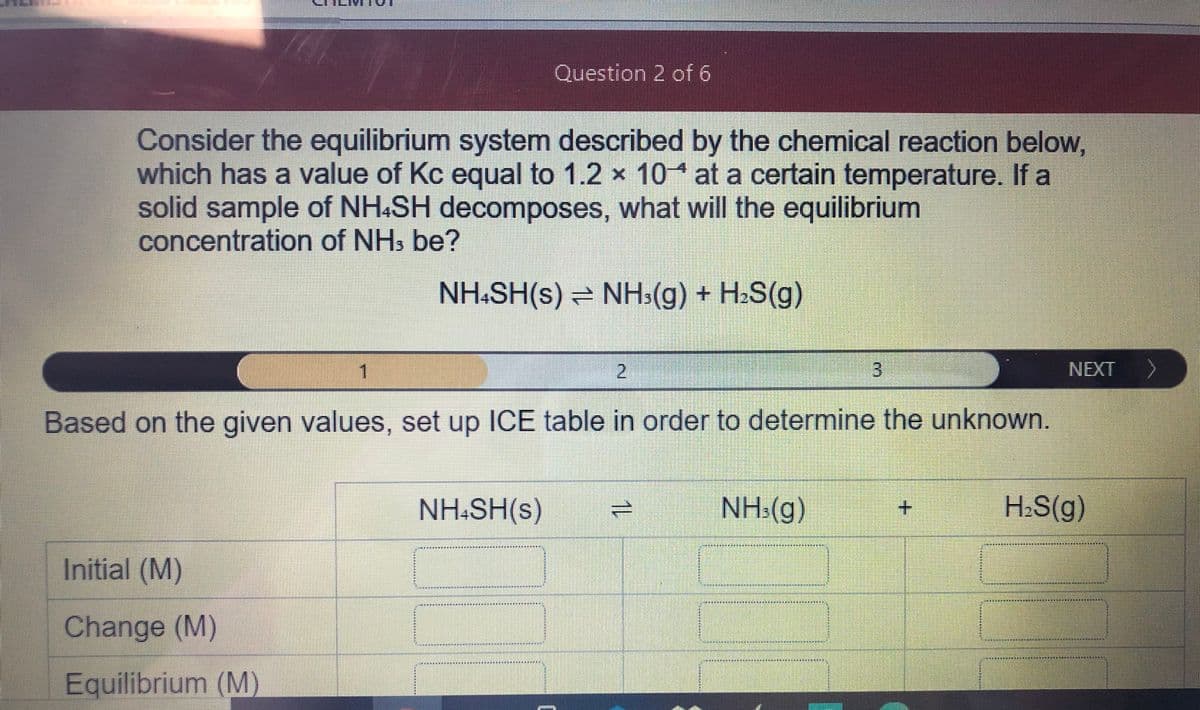
Transcribed Image Text:Question 2 of 6
Consider the equilibrium system described by the chemical reaction below,
which has a value of Kc equal to 1.2 x 10* at a certain temperature. If a
solid sample of NH&SH decomposes, what will the equilibrium
concentration of NHs be?
NH:SH(s) NH:(g) + H2S(g)
1.
2.
NEXT
へ
Based on the given values, set up ICE table in order to determine the unknown.
NH:SH(s)
NH:(g)
H:S(g)
**************t
Initial (M)
其刻其其其
***
其其
其
Change (M)
Equilibrium (M)
3.
1L
Expert Solution
Step 1-4
The value of Kc for the reaction of NH4SH is given and the reaction is
The ICE tables give us information about the concentration of the compounds. In the ICE, I stand for the initial concentration, C is change in concentration and E is equilibrium concentration.
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
