Consider the following chemical equilibrium: HCOO- (aq) + H2O(aq) = HCOOH (aq) + OH- (aq). Which of the following graphs represents the perturbation on the system and the change of pH when HCI is added to the container? III PH PH ↑ time time IV PH Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer. a b || C III d IV pH time time Open Your answer
Consider the following chemical equilibrium: HCOO- (aq) + H2O(aq) = HCOOH (aq) + OH- (aq). Which of the following graphs represents the perturbation on the system and the change of pH when HCI is added to the container? III PH PH ↑ time time IV PH Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer. a b || C III d IV pH time time Open Your answer
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter12: Chemical Equilibrium
Section: Chapter Questions
Problem 12.65PAE
Related questions
Question
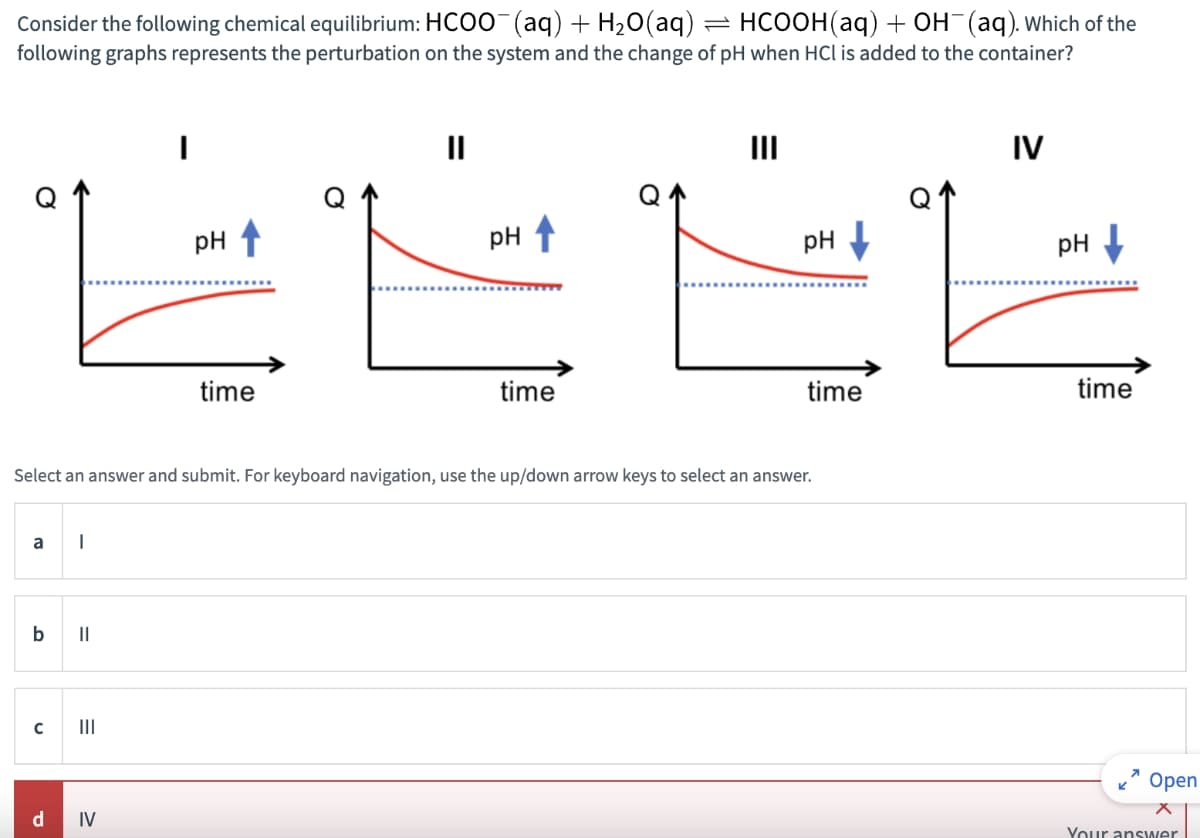
Transcribed Image Text:Consider the following chemical equilibrium: HCOO- (aq) + H2O(aq)
=
HCOOH (aq) + OH- (aq). Which of the
following graphs represents the perturbation on the system and the change of pH when HCI is added to the container?
III
PH
PH ↑
time
time
IV
PH
Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer.
a
b ||
C
III
d
IV
pH
time
time
Open
Your answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 1 steps

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER