Q: Calculate the energy required to heat 744.0mg of silver from −5.6°C to 19.1°C. Assume the specific…
A: The -5.60C and 19.10C are equal to 267.55 K and 292.25 K respectively. The mass of 744 mg of silver…
Q: An automobile engine provides 585 Joules of work to push the pistons and generates 2368 Joules of…
A: The first law of thermodynamics states that “the energy of an isolated system is constant”. It is…
Q: An automobile engine provides 510 Joules of work to push the pistons and generates 2202 Joules of…
A: Given : work done by system = 510 J Heat release = 2202 J We know ∆U = Q + W
Q: An automobile engine provides 545 Joules of work to push the pistons and generates 2395 Joules of…
A:
Q: Consider the following reaction equation of combustion of glucose, C6H12O6: a. Calculate the…
A: Mass of Glucose = 5.25 g C6H12O6 (s) + 6O2(g) ------> 6CO2(g) + 6H2O(g)
Q: 9. The evaporation of water is endothermic: H2O (1) → H2O (g) AH°rxn = 44.01 kJ/mol What minimum…
A: H°rxn = 44.01 kJ / mol
Q: A chemical reaction is run in which 209 Joules of heat are generated and 540 Joules of work are done…
A: According to first law of thermodynamics change in internal energy is equal to sum of change is heat…
Q: Are the following processes exothermic or endothermic? a. the combustion of gasoline in a car engine…
A: Exothermic reaction: The reaction or process in which energy is released is known as exothermic…
Q: Calculate the change in internal energy for a system that is giving off 45.0 kJ of heat and is…
A: According to the second law of thermodynamics, change in internal energy is related to heat and work…
Q: A scientist measures the standard enthalpy change for the following reaction to be 7.8 kJ : Fe(s) +…
A: Consider the given information is as follows; Fes + 2 HClaq → FeCl2s + H2g…
Q: Find the standard enthalpy of formation of ethylene, C2H4(g), given the following data:
A: The given problem relates to Hess's law, which states that the amount of heat evolved or absorbed in…
Q: A scientist measures the standard enthalpy change for the following reaction to be 80.6…
A: Given, Standard enthalpy change = 80.6kJ. The given reaction is
Q: The hypothetical element X and an oxide of X undergo the following reactions with oxygen, with the…
A: According to Hess law the standard enthalpy of reaction is equal to the sum of enthalpies of…
Q: Benzene (C6H6) burns in air to produce carbon dioxide and liquid water. How much heat is released…
A:
Q: The enthalpy change for Photosynthesis can be represented as the following thermochemical equation.…
A: Photosynthesis is the chemical process where the plant absorbs sunlight and converts into a glucose…
Q: A 6.55 g sample of aniline (C,H5NH2, molar mass = 93.13 g/mol) was combusted in a bomb calorimeter.…
A:
Q: Four different substance each have identical masses. The specific heat capacities of these four…
A: The quantity of heat required to alter the temperature of a unit mass of a substance by one degree…
Q: Ozone, O3(g), forms from oxygen, O2(g), by an endothermic process. Ultraviolet radiation is the…
A: the standared enthalpy of formation of ozone is equal to the enthaply of formation of 1 mole of…
Q: Explain the difference between the change in internal energy and the change in enthalpy of a…
A: Change in internal energy (∆U): Internal energy is the sum of Potential energy and the work done of…
Q: The molar heat capacity of silver is 25.35 J/mol Celcius. How much energy would it take to raise the…
A: The relationship between heat required and specific heat of a substance is given below.
Q: What is the change in the internal energy of the system if the surroundings releases 300J of heat…
A:
Q: An automobile engine provides 646 Joules of work to push the pistons and generates 2258 Joules of…
A: According to the thermodynamics law ∆U = Q + W Given Work done on the piston = - 646 J Heat…
Q: The internal energy of a gas decreases by 1.65kj when it transfers 1.87kj of energy in the form of…
A: Interpretation: The work done on the gas by the surroundings is to be determined. Concept…
Q: 1. The combustion of a sample of aluminium produces 0.25 mol of aluminium oxide and releases 419 kJ…
A: The combustion reaction of aluminum is given by: Als + 34O2g → 12Al2O3s The enthalpy change in…
Q: A chemist carefully measures the amount of heat needed to raise the temperature of a 289.0mg sample…
A: given: mass = 289 mg = 0.289 g T2 = 40oC T1 = 22.1°C Q = 8.75 J molar mass of C4H8O2 = 88.11 g/mol…
Q: An engineering student wants to study the heat transfer between a 50 g of heated block of iron (cFe…
A:
Q: Calculate the standard molar enthalpy of formation of nitrogen monoxide, NO, given the following…
A: This is an application of Hess's law
Q: It takes 183 J of energy to raise the temperature of 118.4 g of zinc from 23.61°C to 27.58°C.…
A: Given, Heat energy required = 183J. Mass of zinc = 118.4g. Initial temperature = 23.610C. Final…
Q: Consider the following four cases: (i) A chemical process in which heat is absorbed, (ii) A change…
A: Given here four cases and we are asked in how many of these cases does the internal energy of the…
Q: A chemical reaction is run in which 460 Joules of heat are absorbed and 555 Joules of work is done…
A: Given: Amount of heat absorbed = 460 Joules Work done by the system = 555 Joules We have to…
Q: A scientist measures the standard enthalpy change for the following reaction to be 84.6 kJ :…
A: Known Value:∆Hf°NH4Cl (aq) = Standard enthapy of formation of NH4Cl (aq)…
Q: A scientist measures the standard enthalpy change for the following reaction to be 11.0 kJ…
A: Standard Enthalpy change of a reaction is the sum of the standard enthalpies of products minus the…
Q: A chemist carefully measures the amount of heat needed to raise the temperature of a 412.0 mg sample…
A:
Q: The hypothetical element X and an oxide of X undergo the following reactions with oxygen, with the…
A: Answer: -150 KJ Solution:
Q: According to the First Law of Thermodynamics, a system which absorbs 40 J of heat and produces 20 J…
A:
Q: A scientist measures the standard enthalpy change for the following reaction to be 209.4 kJ : CHĄ(g)…
A: Given chemical equation: CH4g+H2Og→3H2g+COg
Q: Natural gas when allowed to expand from a volume of 5.2 L to a volume of 9.5 L. While the process of…
A: Since the relationship between the work and change in volume is given by W = -Pext X (Vf - Vi )…
Q: the difference between changes in enthalpy and internal energy for the explosion of…
A: Nbh
Q: Consider this reaction: C6H12O6 + 60₂ à 6CO₂ + 6H₂O. What is the standard enthalpy AH°rxn given the…
A:
Q: Determine the molar enthalpy of combustion of an unknown fuel if a 2.75 g sample increased the…
A:
Q: Consider a mixture of air and gasoline vapor in a cylinder with a piston. The original volume is 36…
A:
Q: 2H2 + 2 02 --> 2H20, + 296 kJ is an example of a(n)
A: Thermodynamically Reactions are classified as - Exothermic and endothermic. (1) Exothermic Reactions…
Q: A chemist carefully measures the amount of heat needed to raise the temperature of a 2.00kg sample…
A: Given dataMass of C2H2Cl2 = 2.0kgTotal heat = 2.25x104 JTemperature difference = (16.5 - 6.3)°C
Q: if the specific heat capacity of iron (Cs ) is 0.499 J/g * degree celsius. what is the heat capacity…
A: Number of mole=mass/molar mass
Q: Benzene (C6H6) burns in air to produce carbon dioxide and liquid water. Calculate the heat released…
A: to solve the question we should know the enthalpy of formation of all the reactant and product…
Q: The work done to compress gas is 83.0 J. As a result, 33.0 J of heat is given off to the…
A: According to the 1st law of thermodynamics, the relation between change in internal energy, work…
Q: Determine the change in the internal energy of a system if 5.5 x 107 kJ of heat is removed from it…
A: Given Data- q = -5.5×107 kJ (Heat is removed) W = +2.5×107 kJ (work done on the system)
Q: The work done to compress a gas is 69.0 J. As a result, 37.0 J of heat is given off to the…
A: Given, Heat given off q = 37 J , work done w = 69 J , change on energy ∆U = ?
Q: 8. Liquid ethanol, C,H;OH(1), has been proposed as an alternative fuel. Calculate the standard…
A:
Q: A scientist measures the standard enthalpy change for the following reaction to be 79.4 kJ:…
A: Consider the given reaction is as follows; NH4Cl(aq) → NH3(g) + HCl(aq)…
Consider the following
C3H8(g) + 5 O2(g) → 3 CO2(g) +4 H2O(g)
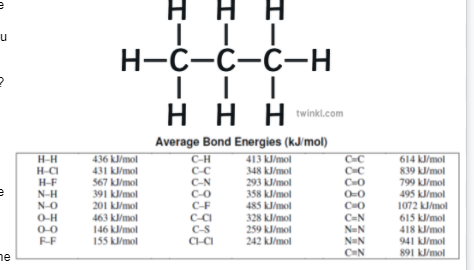
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

- Bond Energies (kJ/mol) Bond Bond Energy Bond Bond Energy Bond Bond Energy H–H 436 C–S 260 F–Cl 255 H–C 415 C–Cl 330 F–Br 235 H–N 390 C–Br 275 Si–Si 230 H–O 464 C–I 240 Si–P 215 H–F 569 N–N 160 Si–S 225 H–Si 395 N=NN=N 418 Si–Cl 359 H–P 320 N≡NN≡N 946 Si–Br 290 H–S 340 N–O 200 Si–I 215 H–Cl 432 N–F 270 P–P 215 H–Br 370 N–P 210 P–S 230 H–I 295 N–Cl 200 P–Cl 330 C–C 345 N–Br 245 P–Br 270 C=CC=C 611 O–O 140 P–I 215 C≡CC≡C 837 O=OO=O 498 S–S 215 C–N 290 O–F 160 S–Cl 250 C=NC=N 615 O–Si 370 S–Br 215 C≡NC≡N 891 O–P 350 Cl–Cl 243 C–O 350 O–Cl 205 Cl–Br 220 C=OC=O 741 O–I 200 Cl–I 210 C≡OC≡O 1080 F–F 160 Br–Br 190 C–F 439 F–Si 540 Br–I 180 C–Si 360 F–P 489 I–I 150 C–P 265 F–S 285 Molecular ekacagine combines with ethylene to form diekaethylene (C2H2Ec2) according to the reaction below: Examine the bond energies in Table 9.3 of your textbook. Shuri’s undergrads measured the new bond enthalpies: C-Ec bond energy is 220 kJ/mol and the…Given the following thermodynamic data, calculate the lattice energy of LiCl:ΔH°f[LiCl(s)] = -409 kJ/molΔH°sublimation [Li] = 161 kJ/molBond energy [Cl-Cl] = 243 kJ/molIE1 (Li) = 520 kJ/molEA1 (Cl) = -349 kJ/mol -1682 kJ/mol -984 kJ/mol -1560 kJ/mol -862 kJ/mol -1213 kJ/molUse the bond energies provided to estimate ΔH°rxn for the reaction below.XeF2 + 2 F2 → XeF6ΔH°rxn = ? Bond Bond Energy (kJ/mol) Xe-F 147 F-F 159
- Some of bond energies (kJ/mol)H-H: 436C-H: 416C-C single bond: 356C-C double bond: 598C-C triple bond: 813N-H: 391C-N: 285N-N: 160N-N triple bond: 946O-H: 467C-O single bond: 336C-O double bond: 803N-O: 201O-O double bond: 498Bond Bond Energy (kJ/mol) Bond Bond Energy (kJ/mol) C-C 347 C=O (in CO2) 799 C=C 614 C=O 745 C≡C 839 C≡O 1070 C-H 413 O=O 498 O-H 467 N-H 391 H-H 432 N≡N 945 C-O 358 N-Cl 200 C-N 305 N-O 201 C-Cl 339 C≡N 891 Cl-Cl 243 S-Cl 250 S=O 535 S-S 215 S-O 364 S-H 339 Using the table of bond energies above, estimate the enthalpy change (kJ) for the following reaction: CH4+2O2⟶CO2+2H2OThe Bond energy of C2(g) is 599 kJ/mol and for F2 is 154 kJ/mol. Use MO theory to justify the difference.
- Use the table below to determine ΔH for the following reactionN 2H 4(g) + H 2O(g) ↔ NH 2OH(g) + NH 3(g) Bond Energies, kJ/mol Single Bonds H C N O S F Cl Br I H 432 C 411 346 N 386 305 167 O 459 358 201 142 S 363 272 --- --- 286 F 565 485 283 190 284 155 Cl 428 327 313 218 255 249 240 Br 362 285 243 201 217 249 216 190 I 295 213 --- 201 --- 278 208 175 149 Multiple Bonds C=C 602 C=N 615 C=O 799 C≡C 835 C≡N 887 C≡O 1072 N=N 418 N=O 607 S=O (in SO2) 532 N≡N 942 O2 494 S=O (in SO3) 469Which of the following electron configuration pairs would most likely result in covalent bonding? A. 1s2 2s2 2p5 and 1s2 2s2 2p6 B. 1s2 2s2 2p5 and 1s2 2s2 2p5 C. 1s2 2s2 and [Ar] 4s1 D. [Ne] 3s1 and [Ne] 3s1Consider the following reaction:H2 (g) + O2 (g) --> H2O2 (g) (Delta)H = -153 kJGiven that the H2 bond energy is 432 kJ/mol, the O2 bond energy is 495 kJ/mol, and the OH bond energy is 467 kJ/mol, estimate the bond energy for the oxygen-oxygen single bond (in kJ/mol).
- Suppose there is an element X which occurs naturally as X2(g).X2(g) + 2O2(g) → X2O4(g)ΔHof of O(g) is 249 kJ/molΔHof of X(g) is 458.5 kJ/molΔHof of X2O4(g) is 31 kJ/molThe X-X single bond energy is 116 kJ/molUse the above data to estimate the average bond energy in X2O4. Give your answer to the nearest 1 kJ/mol.2.7 Which of the following electron configurations belongs to an atom that is most likely to be involved in an ionic bond? 1s22s22p63s2 1s22s22p63s23p6 1s22s22p6 1s22s22p63s23p3Is this correct? (I think the degree symbol represents a triple bond)

