A chemist carefully measures the amount of heat needed to raise the temperature of a 289.0mg sample of C4H8O2 from 22.1°C to 40.0°C. The experiment shows that 8.75J of heat are needed. What can the chemist report for the molar heat capacity of C4H8O2? Be sure your answer has the correct number of significant digits.
A chemist carefully measures the amount of heat needed to raise the temperature of a 289.0mg sample of C4H8O2 from 22.1°C to 40.0°C. The experiment shows that 8.75J of heat are needed. What can the chemist report for the molar heat capacity of C4H8O2? Be sure your answer has the correct number of significant digits.
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter9: Energy And Chemistry
Section: Chapter Questions
Problem 9.50PAE: 9.50 When a 13.0-g sample of NaOH(s) dissolves in 400.0 mL of water in a coffee cup calorimeter, the...
Related questions
Question
A chemist carefully measures the amount of heat needed to raise the temperature of a 289.0mg sample of C4H8O2 from 22.1°C to 40.0°C. The experiment shows that 8.75J of heat are needed. What can the chemist report for the molar heat capacity of C4H8O2? Be sure your answer has the correct number of significant digits.
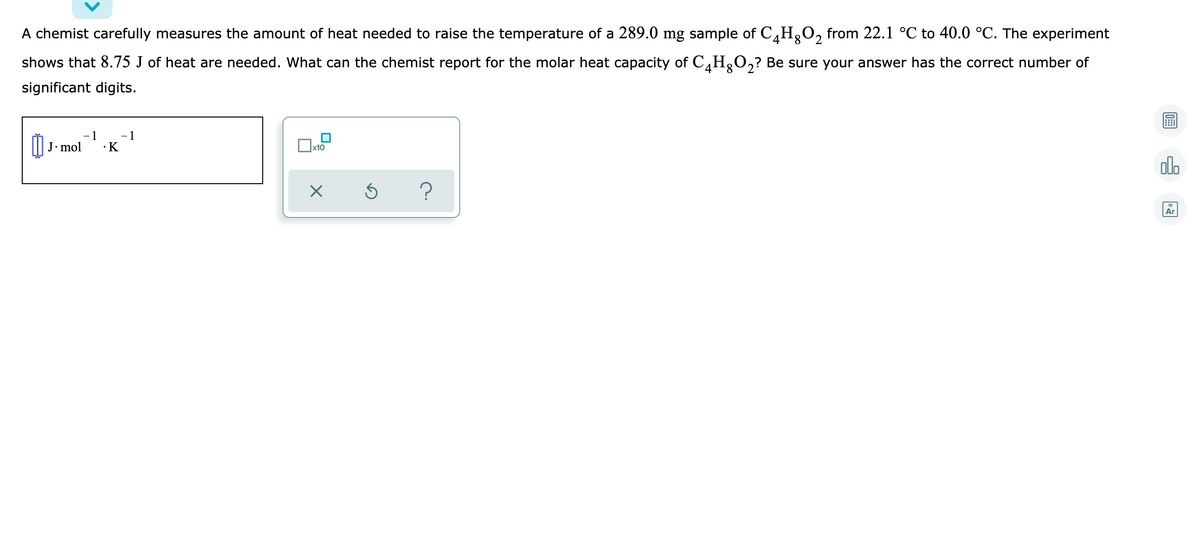
Transcribed Image Text:A chemist carefully measures the amount of heat needed to raise the temperature of a 289.0 mg sample of C,HgO, from 22.1 °C to 40.0 °C. The experiment
shows that 8.75 J of heat are needed. What can the chemist report for the molar heat capacity of C,H,O,? Be sure your answer has the correct number of
significant digits.
J. mol
1
- 1
·K
x10
olo
Ar
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
