Consider the following reaction: 2S03(g) 2s02(g) + O2(g) If 0.136 moles of SO3(g), 0.542 moles of SO2, and 0.254 moles of O2 are at equilibrium in a 16.4 L container at 1.29×10³ K, the value of the equilibrium constant, Ke, is
Consider the following reaction: 2S03(g) 2s02(g) + O2(g) If 0.136 moles of SO3(g), 0.542 moles of SO2, and 0.254 moles of O2 are at equilibrium in a 16.4 L container at 1.29×10³ K, the value of the equilibrium constant, Ke, is
Chemistry for Engineering Students
3rd Edition
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter12: Chemical Equilibrium
Section: Chapter Questions
Problem 12.54PAE: Consider the following system: 4NH 3 ( g )+ 3O 2 ( g ) N 2 ( g )+ 6H 2 O( l ) H=1530.4kJ (a) How...
Related questions
Question
100%
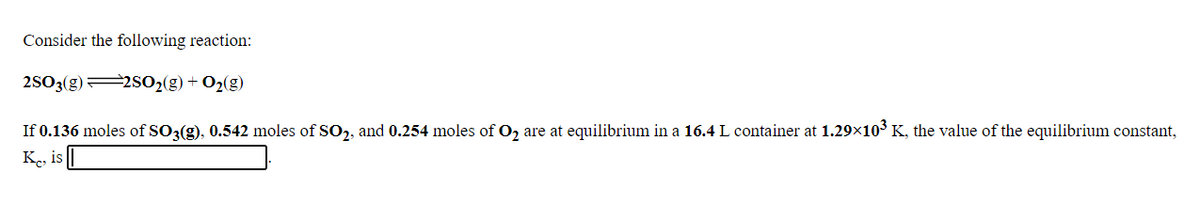
Transcribed Image Text:Consider the following reaction:
2SO3(g)2S02(g) + O2(g)
If 0.136 moles of SO3(g), 0.542 moles of SO,, and 0.254 moles of O, are at equilibrium in a 16.4 L container at 1.29×103 K, the value of the equilibrium constant,
Ke, is
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning