Consider the orbitals shown here in outline. 00 (x) (y) (z) (a) What is the maximum number of electrons contained in an orbital of type (x)? Of type (y)? Of type (z)? (b) How many orbitals of type (x) are found in a shell with n = 2? How many of type (y)? How many of type (z)? (c) Write a set of quantum numbers for an electron in an orbital of type (x) in a shell with n = 4. Of an orbital of type (y) in a shell withn=2. Of an orbital of type (z) in a shell with n = 3. (d) What is the smallest possible n value for an orbital of type (x)? Of type (y)? Of type (z)? (e) What are the possible l and mị values for an orbital of type (x)? Of type (y)? Of type (z)?
Consider the orbitals shown here in outline. 00 (x) (y) (z) (a) What is the maximum number of electrons contained in an orbital of type (x)? Of type (y)? Of type (z)? (b) How many orbitals of type (x) are found in a shell with n = 2? How many of type (y)? How many of type (z)? (c) Write a set of quantum numbers for an electron in an orbital of type (x) in a shell with n = 4. Of an orbital of type (y) in a shell withn=2. Of an orbital of type (z) in a shell with n = 3. (d) What is the smallest possible n value for an orbital of type (x)? Of type (y)? Of type (z)? (e) What are the possible l and mị values for an orbital of type (x)? Of type (y)? Of type (z)?
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter6: Electronic Structure And The Periodic Table
Section: Chapter Questions
Problem 68QAP: Answer the following questions. (a) What characteristic of an atomic orbital does the quantum number...
Related questions
Question
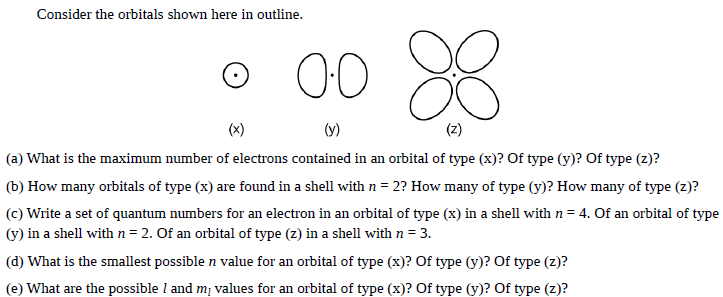
Transcribed Image Text:Consider the orbitals shown here in outline.
00
(x)
(y)
(z)
(a) What is the maximum number of electrons contained in an orbital of type (x)? Of type (y)? Of type (z)?
(b) How many orbitals of type (x) are found in a shell with n = 2? How many of type (y)? How many of type (z)?
(c) Write a set of quantum numbers for an electron in an orbital of type (x) in a shell with n = 4. Of an orbital of type
(y) in a shell withn=2. Of an orbital of type (z) in a shell with n = 3.
(d) What is the smallest possible n value for an orbital of type (x)? Of type (y)? Of type (z)?
(e) What are the possible l and mị values for an orbital of type (x)? Of type (y)? Of type (z)?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 7 steps

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning