Consider the following system at equilibrium where AH° = -111 kJ, and Kc = 0.159, at 723 K. %3D N2(g) + 3H2(g)=2NH3(g) When 0.22 moles of H2(g) are added to the equilibrium system at constant temperature: The value of K. The value of Qc Kc. The reaction mu to restablish equilibrium. to restablish equilibrium. O run in the for Is greater than O run in the rev is equal to O remain the sa ady at equilibrium. is less than The concentration of N2 will
Consider the following system at equilibrium where AH° = -111 kJ, and Kc = 0.159, at 723 K. %3D N2(g) + 3H2(g)=2NH3(g) When 0.22 moles of H2(g) are added to the equilibrium system at constant temperature: The value of K. The value of Qc Kc. The reaction mu to restablish equilibrium. to restablish equilibrium. O run in the for Is greater than O run in the rev is equal to O remain the sa ady at equilibrium. is less than The concentration of N2 will
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter12: Chemical Equilibrium
Section: Chapter Questions
Problem 6QRT: Indicate whether each statement below is true or false. If a statement is false, rewrite it to...
Related questions
Question
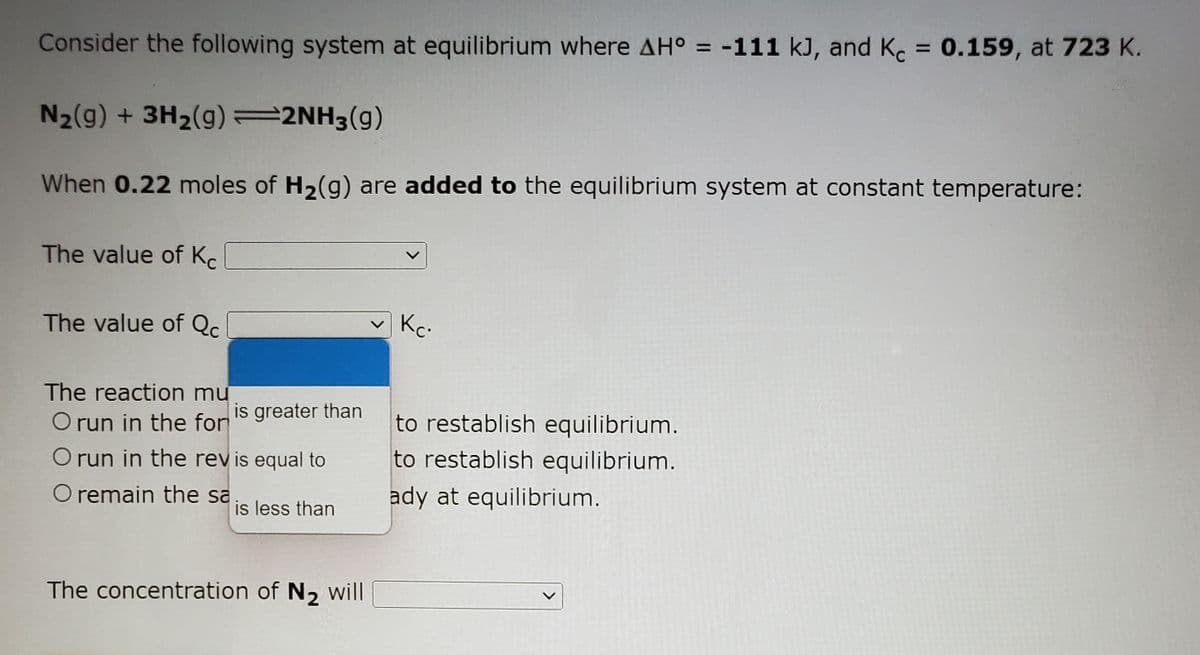
Transcribed Image Text:Consider the following system at equilibrium where AH° = -111 kJ, and K. = 0.159, at 723 K.
%3D
N2(g) + 3H2(g)=2NH3(g)
When 0.22 moles of H2(g) are added to the equilibrium system at constant temperature:
The value of Kc
The value of Qc
Kc.
The reaction mư
O run in the for
is greater than
to restablish equilibrium.
to restablish equilibrium.
O run in the revis equal to
O remain the sa
ady at equilibrium.
is less than
The concentration of N, will
<>
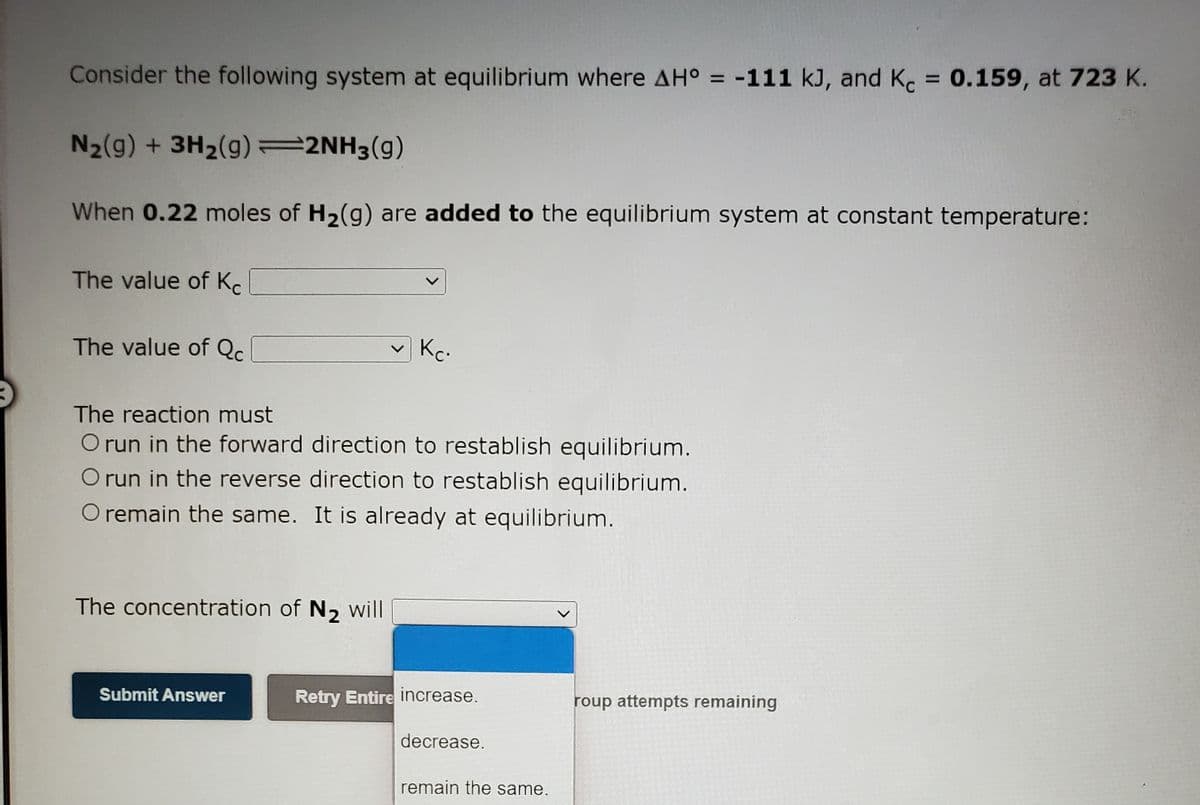
Transcribed Image Text:Consider the following system at equilibrium where AH° = -111 kJ, and K = 0.159, at 723 K.
%3D
%3D
N2(g) + 3H2(g) =2NH3(g)
When 0.22 moles of H2(g) are added to the equilibrium system at constant temperature:
The value of Kc
The value of Qc.
Kc.
The reaction must
O run in the forward direction to restablish equilibrium.
O run in the reverse direction to restablish equilibrium.
O remain the same. It is already at equilibrium.
The concentration of N2 will
Submit Answer
Retry Entire increase.
roup attempts remaining
decrease.
remain the same.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
