explain what the results of this experiment are and the purpose Table 2 Absorbance vs CoCl2 concentration Test Tube Number Cobalt Chloride Concentration (mol/mL) Absorbance at 510 nm 1 0.000 0.000 2 0.009 0.024 3 0.018 0.055 4 0.027 0.085 5 6 0.036 0.045 0.112 0.138
explain what the results of this experiment are and the purpose Table 2 Absorbance vs CoCl2 concentration Test Tube Number Cobalt Chloride Concentration (mol/mL) Absorbance at 510 nm 1 0.000 0.000 2 0.009 0.024 3 0.018 0.055 4 0.027 0.085 5 6 0.036 0.045 0.112 0.138
Biomedical Instrumentation Systems
1st Edition
ISBN:9781133478294
Author:Chatterjee
Publisher:Chatterjee
Chapter5: Biomedical Electronics: Digital
Section: Chapter Questions
Problem 5Q
Related questions
Question
explain what the results of this experiment are and the purpose
Table 2 Absorbance vs CoCl2 concentration
|
Test Tube Number |
Cobalt Chloride Concentration (mol/mL) |
Absorbance at 510 nm |
|
|
|
|
|
1 |
0.000 |
0.000 |
|
2 |
0.009 |
0.024 |
|
3 |
0.018 |
0.055 |
|
4 |
0.027 |
0.085 |
|
5 6 |
0.036 0.045 |
0.112 0.138 |
|
7 |
(Unknown) |
0.088
|
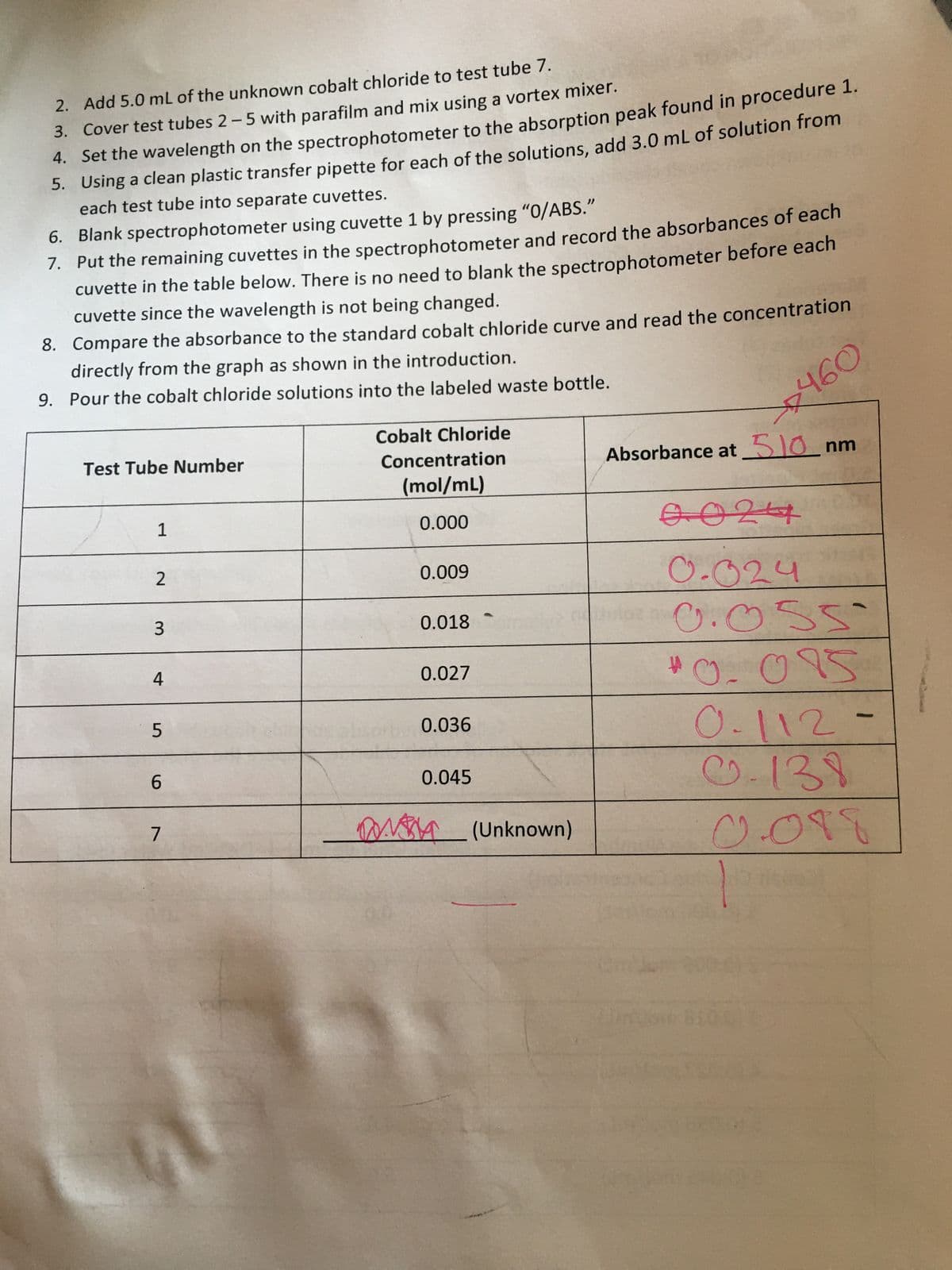
Transcribed Image Text:Add 5.0 mL of the unknown cobalt chloride to test tube 7.
2.
3. Cover test tubes 2-5 with parafilm and mix using a vortex mixer.
Set the wavelength on the spectrophotometer to the absorption peak found in procedure 1.
4.
Using a clean plastic transfer pipette for each of the solutions, add 3.0 mL of solution from
5.
each test tube into separate cuvettes.
6. Blank spectrophotometer using cuvette 1 by pressing "0/ABS."
7.
Put the remaining cuvettes in the spectrophotometer and record the absorbances of each
cuvette in the table below. There is no need to blank the spectrophotometer before each
cuvette since the wavelength is not being changed.
8. Compare the absorbance to the standard cobalt chloride curve and read the concentration
directly from the graph as shown in the introduction.
9. Pour the cobalt chloride solutions into the labeled waste bottle.
Cobalt Chloride
Test Tube Number
Absorbance at 510 nm
Concentration
(mol/mL)
1
0.000
0024
0.009
0.024
0.018
0.055
0.027
#
ตาร
0.015
0.112.
0.036
0.045
0-138
M (Unknown)
COTE
2
3
4
5
6
7
460
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Recommended textbooks for you



