Consider the reaction CO(g) + 2H2(g) CH3OH() at 25°C Calculate value of the equilibrium constant (Kp) for this reaction at 25°C. HINT: first calculate H and S
Consider the reaction CO(g) + 2H2(g) CH3OH() at 25°C Calculate value of the equilibrium constant (Kp) for this reaction at 25°C. HINT: first calculate H and S
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter18: Thermodynamics And Equilibrium
Section: Chapter Questions
Problem 18.60QP: Calculate the standard free-energy change and the equilibrium constant Kp for the following reaction...
Related questions
Question
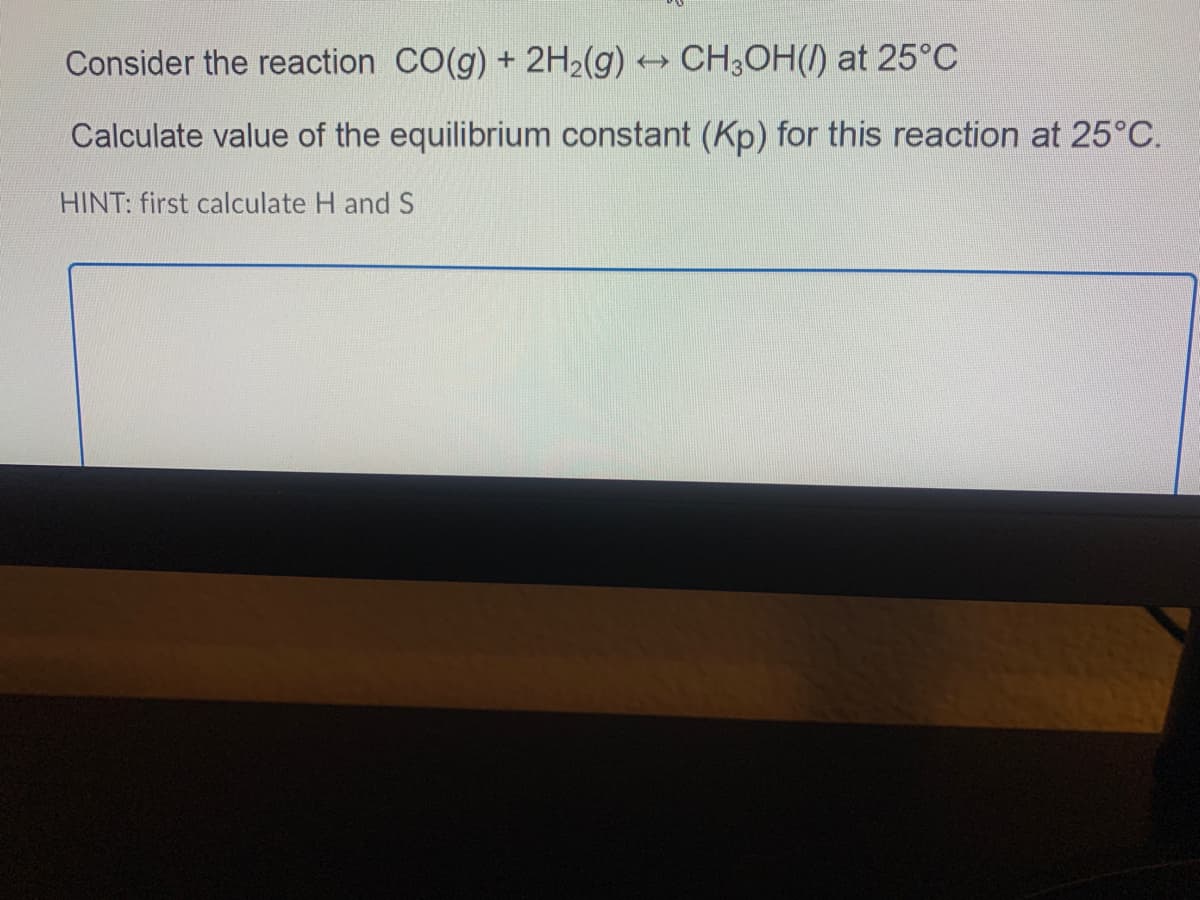
Transcribed Image Text:Consider the reaction CO(g) + 2H2(g) CH3OH(/) at 25°C
Calculate value of the equilibrium constant (Kp) for this reaction at 25°C.
HINT: first calculate H and S
Transcribed Image Text:8:45
A onlcourses.tridenttech.edu
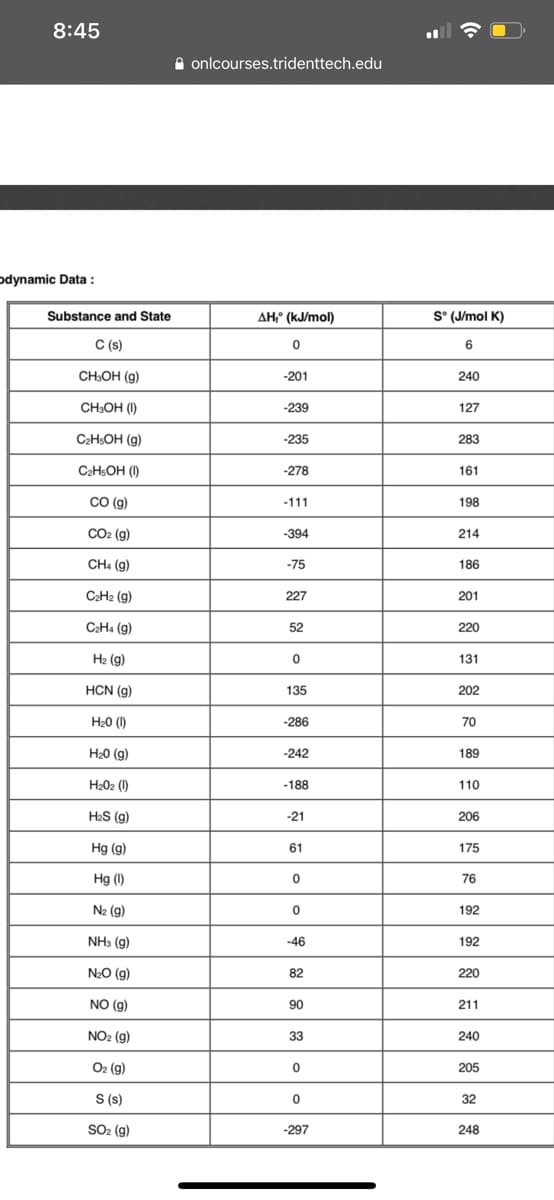
odynamic Data :
Substance and State
AH;" (kJ/mol)
S° (J/mol K)
C (s)
6.
CH:OH (g)
-201
240
CH:OH (I)
-239
127
C2HSOH (g)
-235
283
C2HSOH (I)
-278
161
CO (g)
-111
198
CO2 (g)
-394
214
CH: (g)
-75
186
CaH2 (g)
227
201
CaH4 (g)
52
220
H2 (g)
131
HCN (g)
135
202
H20 (1)
-286
70
H20 (g)
-242
189
H202 (1)
-188
110
H2S (g)
-21
206
Hg (g)
61
175
Hg (I)
76
N2 (g)
192
NH3 (g)
-46
192
N2O (g)
82
220
NO (g)
90
211
NO2 (g)
33
240
O2 (g)
205
S (s)
32
SO2 (g)
-297
248
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning