Consider the reaction: C(s,graphite) + O2(g) (6)*00 Using standard absolute entropies at 298K, calculate the entropy change for the system when 2.36 moles of C(s,graphite) react at standard conditions. AS system J/K %3D
Consider the reaction: C(s,graphite) + O2(g) (6)*00 Using standard absolute entropies at 298K, calculate the entropy change for the system when 2.36 moles of C(s,graphite) react at standard conditions. AS system J/K %3D
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter18: Principles Of Chemical Reactivity: Entropy And Free Energy
Section18.7: The Interplay Of Kinetics And Thermodynamics
Problem 2.2ACP: It has been demonstrated that buckminsterfullerene (C60), another allotrope of carbon (Section 2.3),...
Related questions
Question
100%
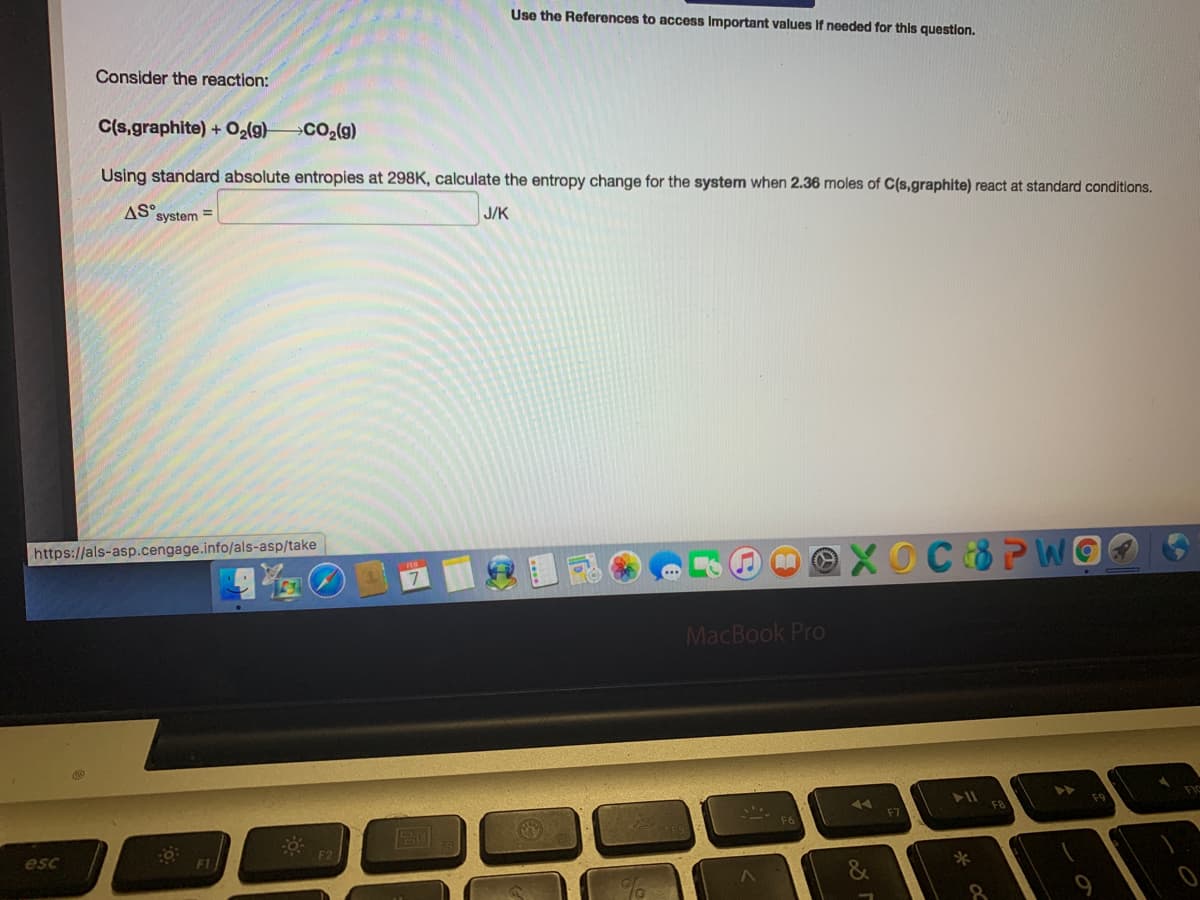
Transcribed Image Text:Use the References to access Important values If needed for this question.
Consider the reaction:
C(s,graphite) + O2(g)
Using standard absolute entropies at 298K, calculate the entropy change for the system when 2.36 moles of C(s,graphite) react at standard conditions.
AS system =
J/K
https://als-asp.cengage.info/als-asp/take
OC 8PWG
MacBook Pro
F8
F7
esc
F2
F1
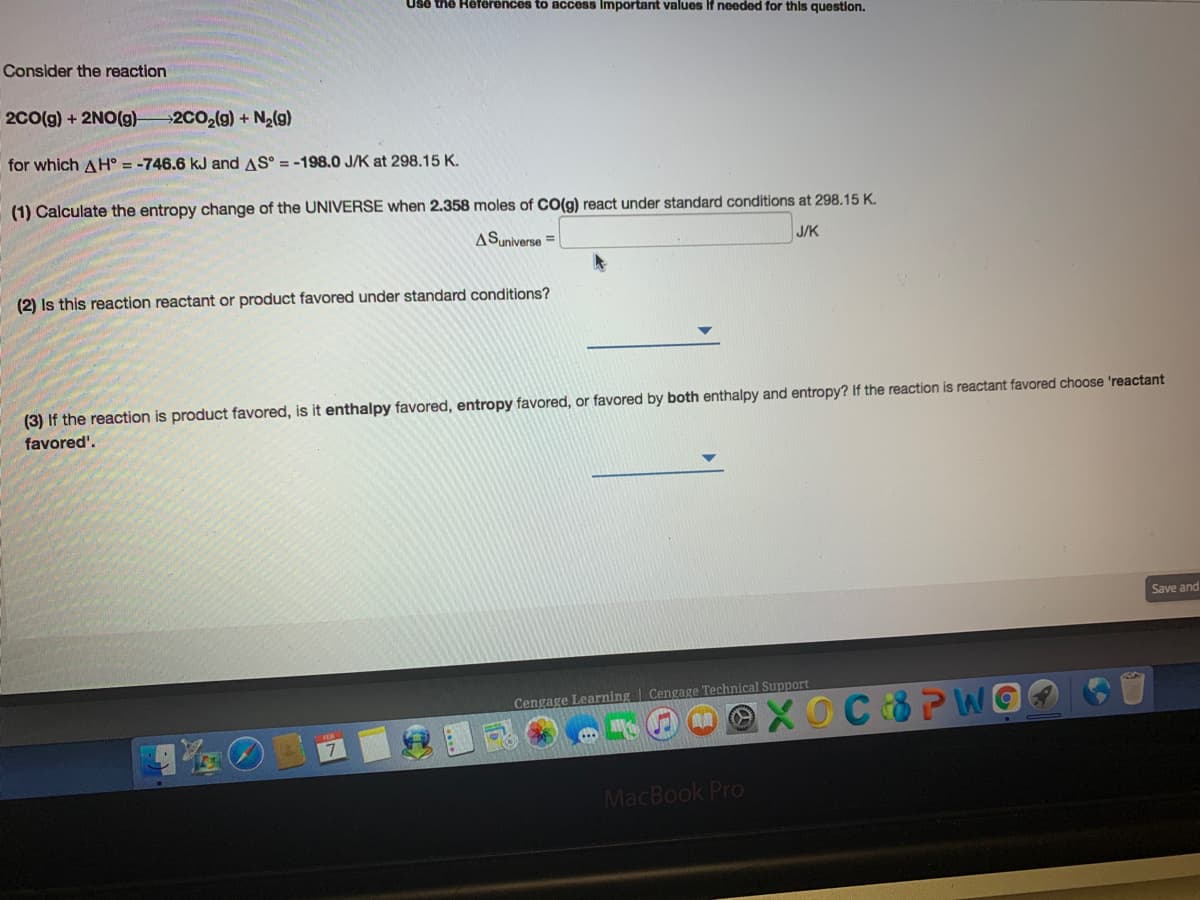
Transcribed Image Text:Use the Heferences to access Important values If needed for this question.
Consider the reaction
2Co(g) + 2NO(g)-
2CO2(g) + N2(g)
for which AH° = -746.6 kJ and AS° = -198.0 J/K at 298.15 K.
(1) Calculate the entropy change of the UNIVERSE when 2.358 moles of CO(g) react under standard conditions at 298.15 K.
ASuniverse =
J/K
(2) Is this reaction reactant or product favored under standard conditions?
(3) If the reaction is product favored, is it enthalpy favored, entropy favored, or favored by both enthalpy and entropy? If the reaction is reactant favored choose 'reactant
favored'.
Save and
Cengage Learning | Cengage Technical Support
C8PWOO
MacBook Pro
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning