Consider the resonance structures of formate. Select the true statemets about the resonance structures. The actual structure of formate is an average of the two resonance forms. Each oxygen atom has a double bond 50% of the time. Each carbon-oxygen bond is somewhere between a single and double bond. The actual structure of formate switches back and forth between the two resonance forms. SA stv MacBook Air
Consider the resonance structures of formate. Select the true statemets about the resonance structures. The actual structure of formate is an average of the two resonance forms. Each oxygen atom has a double bond 50% of the time. Each carbon-oxygen bond is somewhere between a single and double bond. The actual structure of formate switches back and forth between the two resonance forms. SA stv MacBook Air
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter12: Chemical Bonding
Section: Chapter Questions
Problem 36A
Related questions
Question
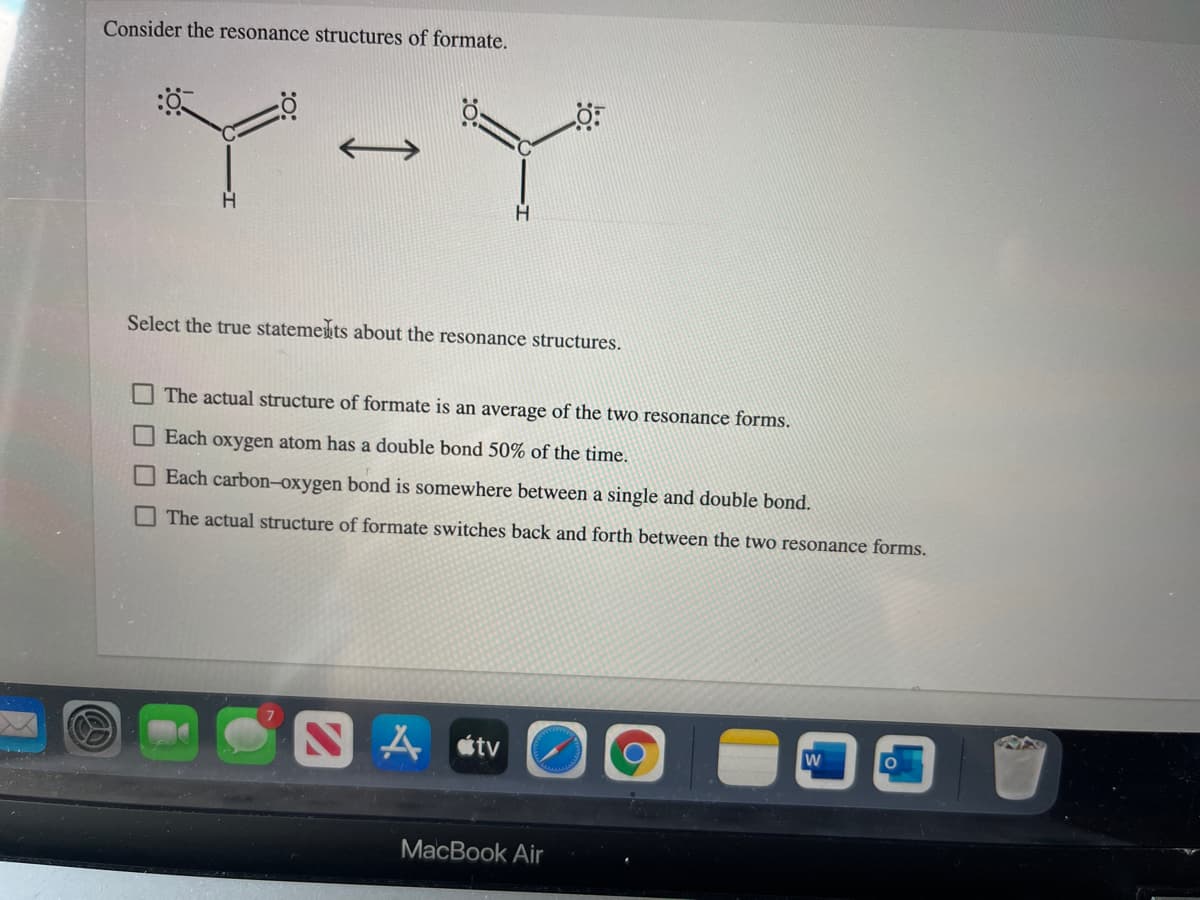
Transcribed Image Text:Consider the resonance structures of formate.
Select the true statemets about the resonance structures.
The actual structure of formate is an average of the two resonance forms.
Each oxygen atom has a double bond 50% of the time.
Each carbon-oxygen bond is somewhere between a single and double bond.
The actual structure of formate switches back and forth between the two resonance forms.
A stv
MacBook Air
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
