Consider the system below at equilibrium at 200°C. 2CI(g) + 2HO9) - 98.6KJ 4HCI(g) + O.g) Addition of catalyst would cause more reactants to form when equilibrium is re-established O True False An example of homogenous equilibrium O D. H.S (ag)+ZHO -S (aq) + ZH.O (aq) 8. 2H90 (a)-2Hg ()+0. (g) O A HO (g) +C(s)- Co (g) +H (g) C CaCO. -Cao (s)+ Co(g)
Consider the system below at equilibrium at 200°C. 2CI(g) + 2HO9) - 98.6KJ 4HCI(g) + O.g) Addition of catalyst would cause more reactants to form when equilibrium is re-established O True False An example of homogenous equilibrium O D. H.S (ag)+ZHO -S (aq) + ZH.O (aq) 8. 2H90 (a)-2Hg ()+0. (g) O A HO (g) +C(s)- Co (g) +H (g) C CaCO. -Cao (s)+ Co(g)
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter12: Chemical Equilibrium
Section: Chapter Questions
Problem 12.22PAE
Related questions
Question
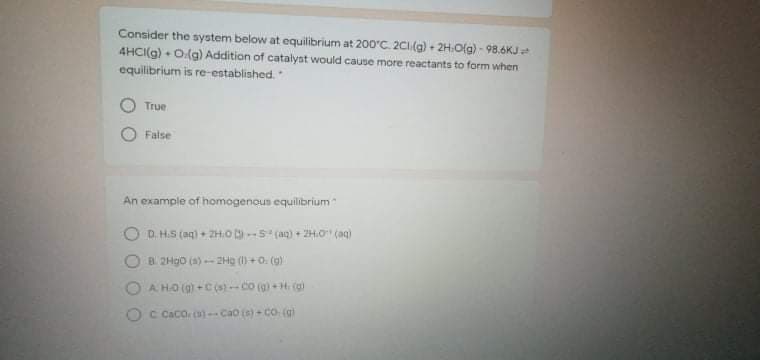
Transcribed Image Text:Consider the system below at equilibrium at 200'C. 2CI(g) + 2H:O(g) - 98.6KJ
4HCI(g) + O(g) Addition of catalyst would cause more reactants to form when
equilibrium is re-established.
True
False
An example of homogenous equilibrium
D. H.S (ag) + ZH.o-S (ag) + ZH.O" (ag)
B 2H90 (a) 2Hg ()+0. (g)
A HO (9) +C(s) -co (g) + H. (g)
OC Caco o)-Cao (s)- Co (g)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning