Consider the table. Metal Tm (K) AHFUS (kJ/mol) Tp (K) AHvap (kJ/mol) Li 454 2.99 1615 134.7 Na 371 2.60 1156 89.6 K 336 2.33 1033 77.1 Rb 312 2.34 956 69 Cs 302 2.10 942 66 Using the data, calculate A.Sfus and ASvap for Cs. K-mol ASyap = K-mol ASfus = TOOLS X10
Consider the table. Metal Tm (K) AHFUS (kJ/mol) Tp (K) AHvap (kJ/mol) Li 454 2.99 1615 134.7 Na 371 2.60 1156 89.6 K 336 2.33 1033 77.1 Rb 312 2.34 956 69 Cs 302 2.10 942 66 Using the data, calculate A.Sfus and ASvap for Cs. K-mol ASyap = K-mol ASfus = TOOLS X10
Chapter31: Introduction To Analytical Separations
Section: Chapter Questions
Problem 31.33QAP
Related questions
Question
I need accurate answer with full details. This is my only chance to get it right.
Transcribed Image Text:Attemp
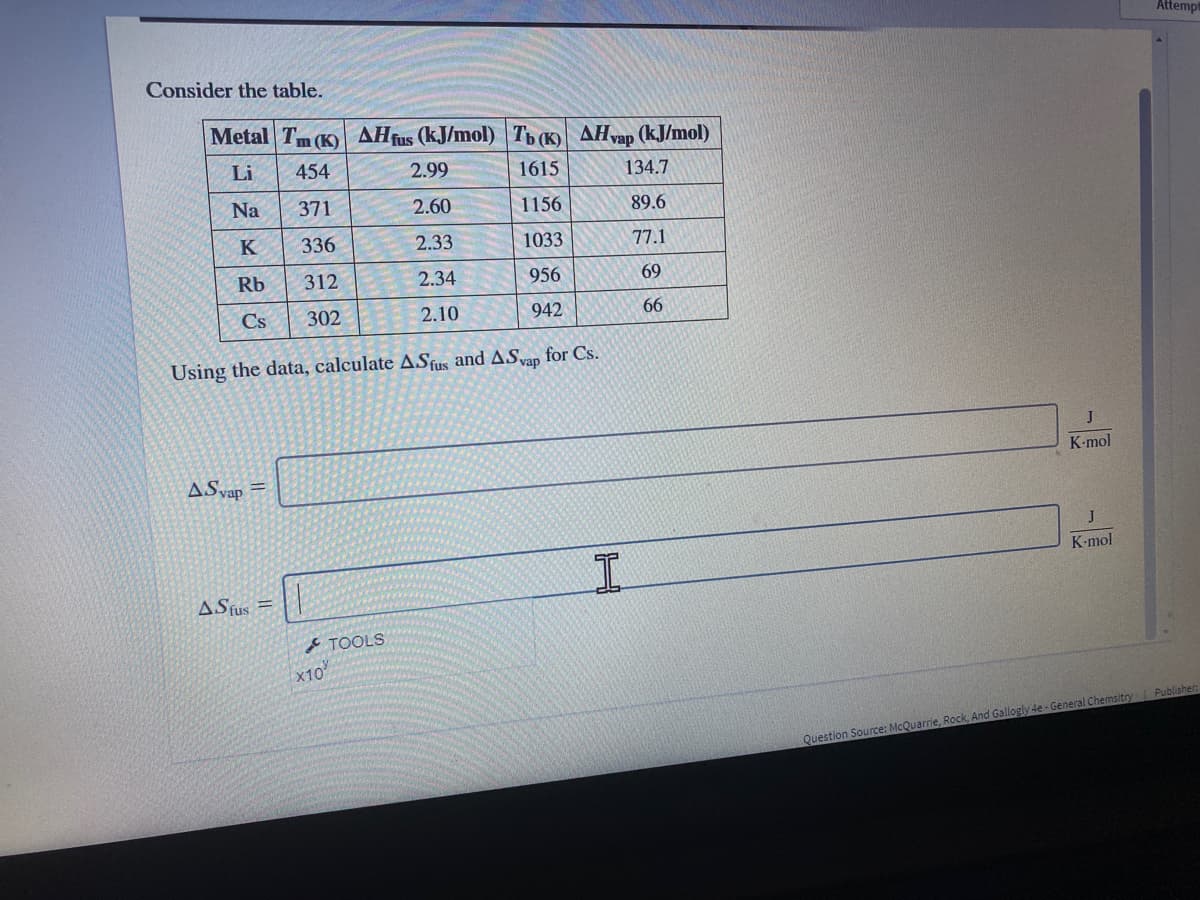
Consider the table.
Metal Tm (K) AHfus (kJ/mol) Tb (K) AHvap (kJ/mol)
Li
454
2.99
1615
134.7
Na
371
2.60
1156
89.6
K
336
2.33
1033
77.1
Rb
312
2.34
956
69
Cs
302
2.10
942
66
Using the data, calculate ASfus and ASap for Cs.
K-mol
ASvap =
K-mol
ASfus
TOOLS
x10
Question Source: McQuarrie, Rock, And Gallogly 4e - General Chemsitry Publisher:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

