Consider two gases, A and B, both at the same temperature. The molar mass of A is MA, the molar mass of B is MB. The graph shows the speed distributions for the A and B molecules. Which dual statement is correct? Probability 0.25 0.20 0.15 50.10 0.05 0.00 0 A B 200 400 600 800 1000 Molecular speed (m/s) OMA = MB. There are more molecules of type A than type B. OMA < MB. A and B have the same average speed. OMA > MB. A and B have the same average speed. OMA MB. A and B have the same average kinetic energy. OMA MB. A and B have the same average kinetic energy.
Consider two gases, A and B, both at the same temperature. The molar mass of A is MA, the molar mass of B is MB. The graph shows the speed distributions for the A and B molecules. Which dual statement is correct? Probability 0.25 0.20 0.15 50.10 0.05 0.00 0 A B 200 400 600 800 1000 Molecular speed (m/s) OMA = MB. There are more molecules of type A than type B. OMA < MB. A and B have the same average speed. OMA > MB. A and B have the same average speed. OMA MB. A and B have the same average kinetic energy. OMA MB. A and B have the same average kinetic energy.
Living By Chemistry: First Edition Textbook
1st Edition
ISBN:9781559539418
Author:Angelica Stacy
Publisher:Angelica Stacy
ChapterU3: Weather: Phase Changes And Behaviour Of Gases
Section: Chapter Questions
Problem 9RE
Related questions
Question
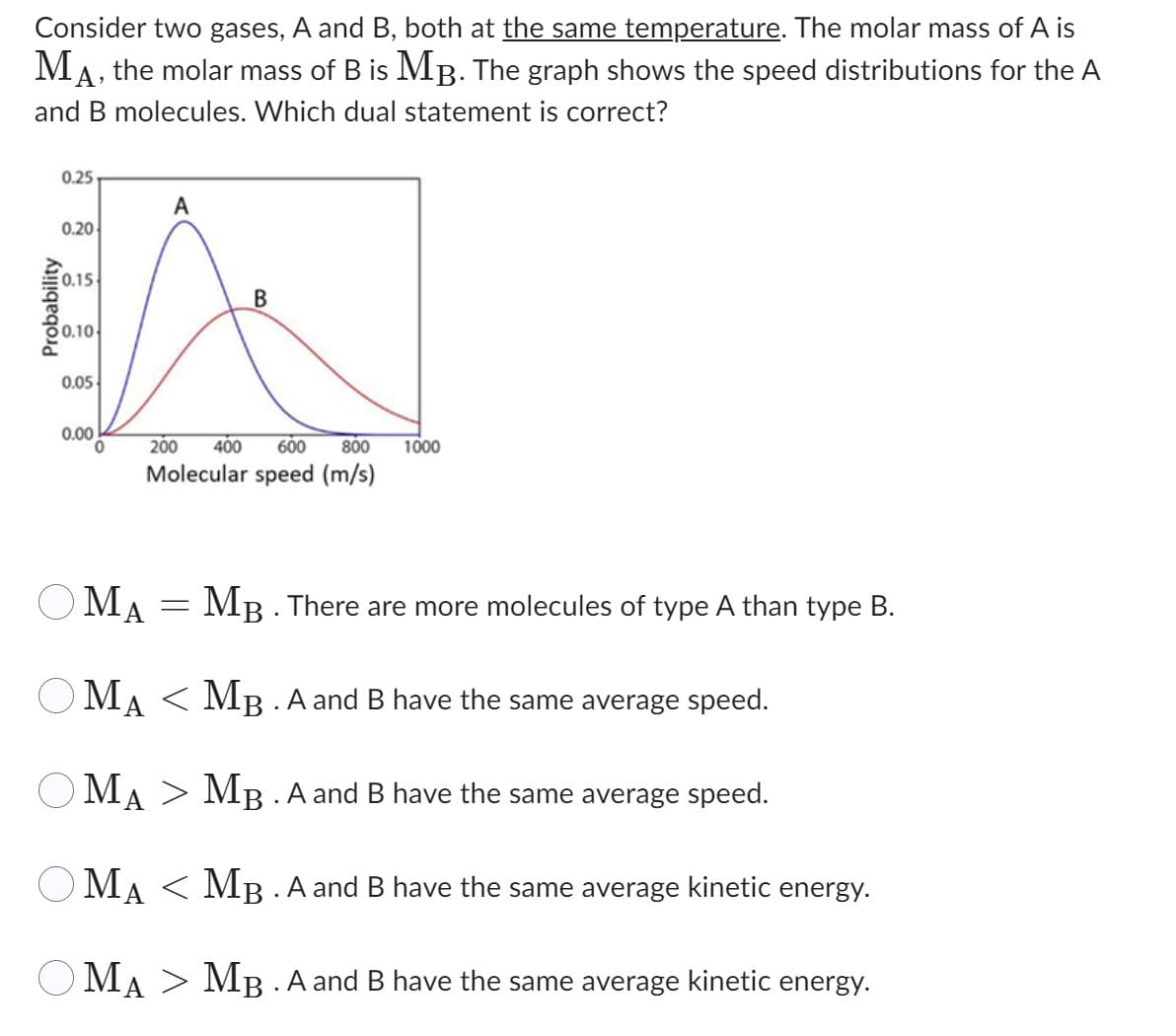
Transcribed Image Text:Consider two gases, A and B, both at the same temperature. The molar mass of A is
MA, the molar mass of B is MB. The graph shows the speed distributions for the A
and B molecules. Which dual statement is correct?
Probability
0.25
0.20
0.15
0.10
0.05
0.00
0
A
B
200 400 600 800 1000
Molecular speed (m/s)
OMA
OMA < MB. A and B have the same average speed.
Ο ΜΑ
MB. A and B have the same average speed.
MAMB. A and B have the same average kinetic energy.
OMA MB. A and B have the same average kinetic energy.
=
MB. There are more molecules of type A than type B.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning