Constants | Periodic Table Chemical energy is released or absorbed from reactions in various forms. The most easily measurable form of energy comes in the form of heat, or enthalpy. The enthalpy of a reaction can be calculated from the heats of formation of the substances involved in the reaction: Part B AHm = En,AH: (products)-E n,AH (reactants) For the reaction given in Part A. how much heat is absorbed when 3.50 mol of A reacts? where n represents the stoichiometric coefficients. Express your answer to three significant figures and include the appropriate units. • View Available Hint(s) Value Units Submit Previous Answers X Incorrect; Try Again; 29 attempts remaining
Constants | Periodic Table Chemical energy is released or absorbed from reactions in various forms. The most easily measurable form of energy comes in the form of heat, or enthalpy. The enthalpy of a reaction can be calculated from the heats of formation of the substances involved in the reaction: Part B AHm = En,AH: (products)-E n,AH (reactants) For the reaction given in Part A. how much heat is absorbed when 3.50 mol of A reacts? where n represents the stoichiometric coefficients. Express your answer to three significant figures and include the appropriate units. • View Available Hint(s) Value Units Submit Previous Answers X Incorrect; Try Again; 29 attempts remaining
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter6: Thermochemisty
Section: Chapter Questions
Problem 6.109QP: A 21.3-mL sample of 0.977 M NaOH is mixed with 29.5 mL of 0.918 M HCl in a coffee-cup calorimeter...
Related questions
Question
100%
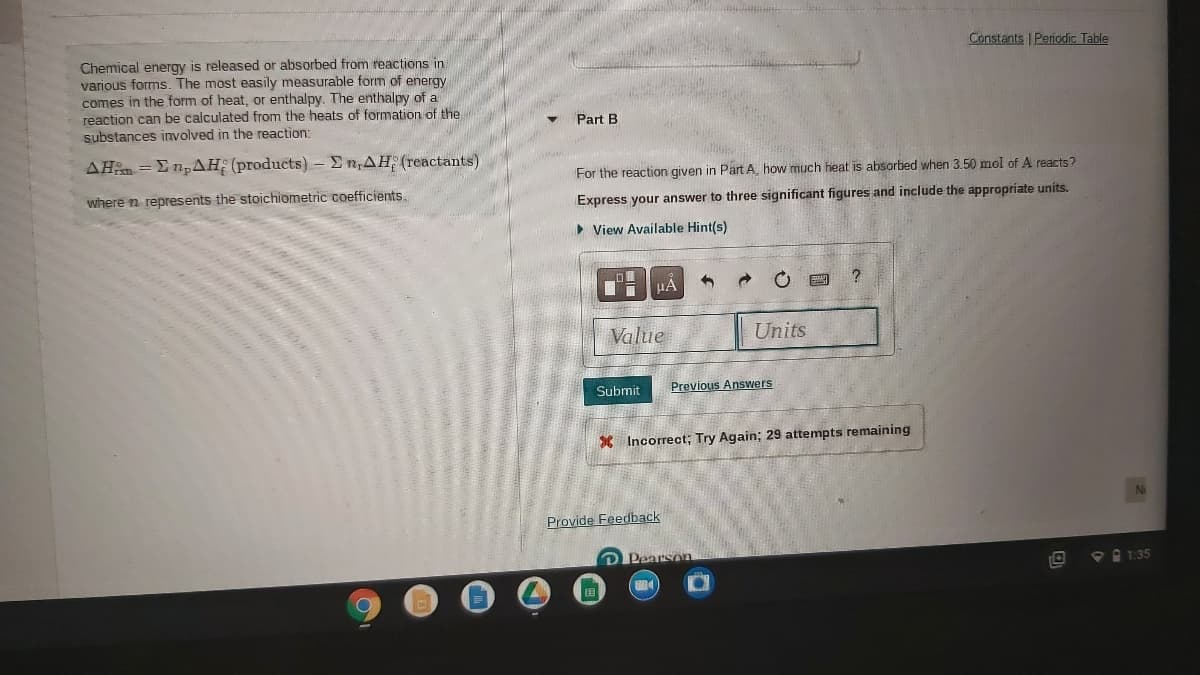
Transcribed Image Text:Constants | Periodic Table
Chemical energy is released or absorbed from reactions in
various forms. The most easily measurable form of energy
comes in the form of heat, or enthalpy. The enthalpy of a
reaction can be calculated from the heats of formation of the
substances involved in the reaction:
Part B
AHm = En,AH: (products)- E n,AH (reactants)
For the reaction given in Part A. how much heat is absorbed when 3.50 mol of A reacts?
where n represents the stoichiometric coefficients.
Express your answer to three significant figures and include the appropriate units.
• View Available Hint(s)
Value
Units
Submit
Previous Answers
X Incorrect; Try Again; 29 attempts remaining
Provide Feedback
Pearson
O 1:35
21
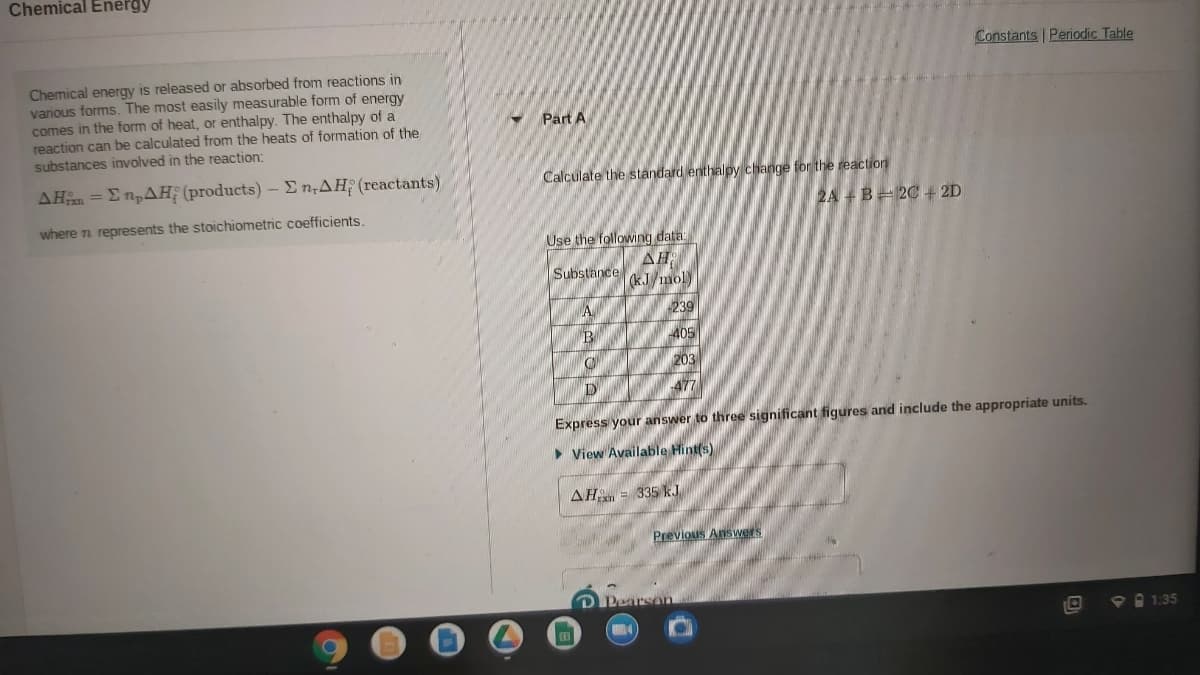
Transcribed Image Text:Chemical Energy
Constants Periodic Table
Chemical energy is released or absorbed from reactions in
various forms. The most easily measurable form of energy
comes in the form of heat, or enthalpy. The enthalpy of a
reaction can be calculated from the heats of formation of the
substances involved in the reaction:
Part A
ΔΗ Σ πρΔΗ (products) -Σn,ΔΗ(reactants)
Calculate the standard enthalpy change for the reaction
2A +B= 2C+ 2D
where n represents the stoichiometric coefficients.
Use the following data
AH
Substance kJ mol)
A
239
405
203
477
Express your answer to three significant figures and include the appropriate units.
> View Available Hint(s)
AH = 335 kJ
Previous Answers
D Pearsan
P 1:35
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning