Respond to questions on the Concept of energy exchange during a chemical reaction using calorimetry A piece of copper metal is initially at 100.0°C. It is dropped into a coffee cup calorimeter containing 50.0 g of water at a temperature of 20.0°C. After stirring, the final temperature of both copper and water is 25.0°C. Assuming no heat losses, and that the specific heat (capacity) of water is 4.18 J/(g K), what is the heat capacity of the copper in J/K? Calculate the amount of heat gained by the water b. Calculate the amount of heat lost by the copper metal c. Calculate heat capacity of the copper in J/K
Respond to questions on the Concept of energy exchange during a chemical reaction using calorimetry A piece of copper metal is initially at 100.0°C. It is dropped into a coffee cup calorimeter containing 50.0 g of water at a temperature of 20.0°C. After stirring, the final temperature of both copper and water is 25.0°C. Assuming no heat losses, and that the specific heat (capacity) of water is 4.18 J/(g K), what is the heat capacity of the copper in J/K? Calculate the amount of heat gained by the water b. Calculate the amount of heat lost by the copper metal c. Calculate heat capacity of the copper in J/K
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter7: Chemical Energy
Section: Chapter Questions
Problem 109AE: A sample of nickel is heated to 99.8C and placed in a coffee-cup calorimeter containing 150.0 g...
Related questions
Question
Pls pls solve accurate and its important pls
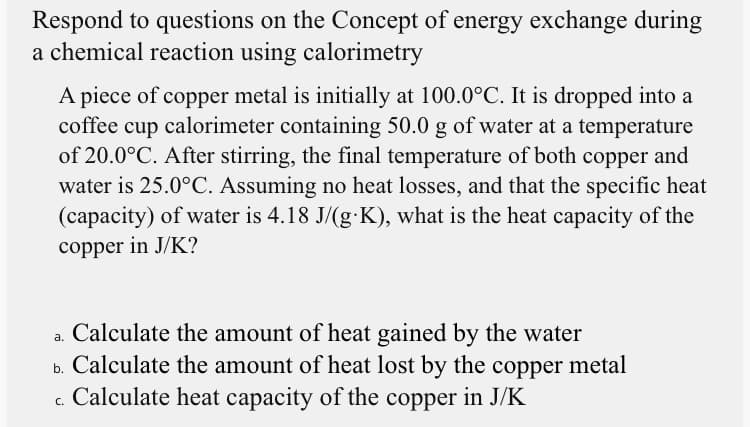
Transcribed Image Text:Respond to questions on the Concept of energy exchange during
a chemical reaction using calorimetry
A piece of copper metal is initially at 100.0°C. It is dropped into a
coffee cup calorimeter containing 50.0 g of water at a temperature
of 20.0°C. After stirring, the final temperature of both copper and
water is 25.0°C. Assuming no heat losses, and that the specific heat
(capacity) of water is 4.18 J/(g-K), what is the heat capacity of the
copper in J/K?
a. Calculate the amount of heat gained by the water
b. Calculate the amount of heat lost by the copper metal
c. Calculate heat capacity of the copper in J/K
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER