Constants Pendc Table You may want to reference (Pages 258 - 262) section 6.4 while completing this problem. Part A An unknown mass of each of the following substances, initially at 25.0°C, absorbs 1960 J of heat, The final temperature is recorded as indicated. Find the mass of each substance. a. Pyrex glass (T = 55.6 °C) Express your answer using two significant figures. ? m = Previous Answers Request Answer
Constants Pendc Table You may want to reference (Pages 258 - 262) section 6.4 while completing this problem. Part A An unknown mass of each of the following substances, initially at 25.0°C, absorbs 1960 J of heat, The final temperature is recorded as indicated. Find the mass of each substance. a. Pyrex glass (T = 55.6 °C) Express your answer using two significant figures. ? m = Previous Answers Request Answer
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter10: Energy
Section: Chapter Questions
Problem 38QAP: In the “Chemistry in Focus” segment Firewalking: Magic or Science?, it is claimed that one reason...
Related questions
Question
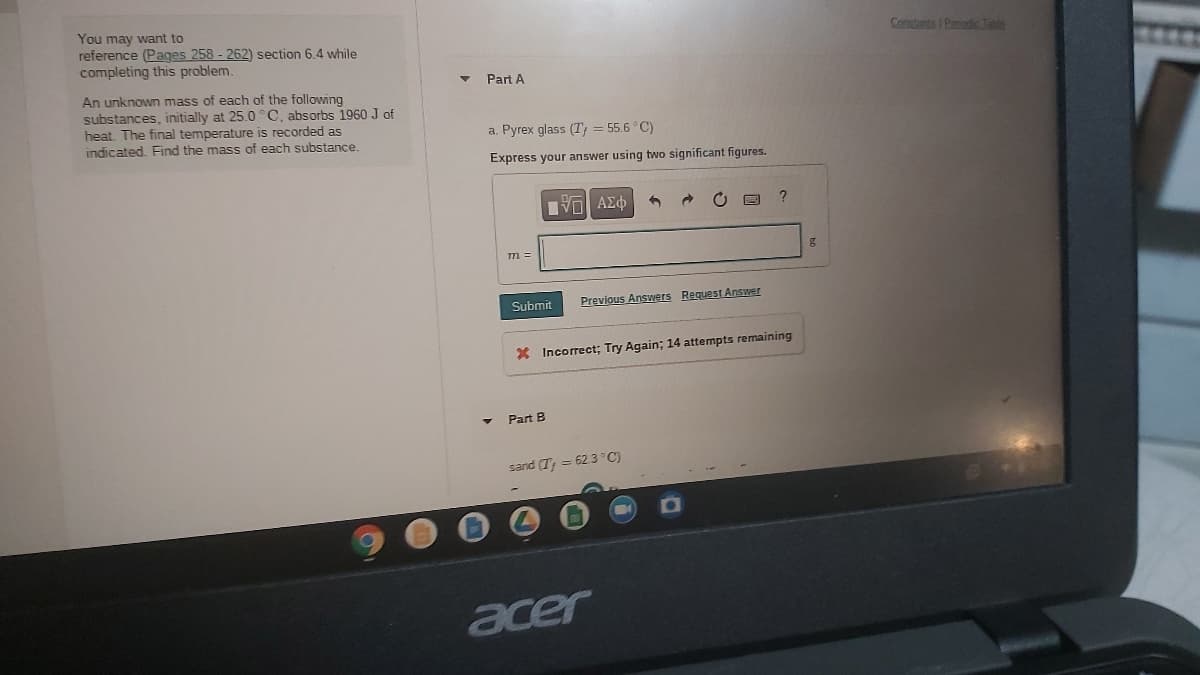
Transcribed Image Text:You may want to
reference (Pages 258 - 262) section 6.4 while
completing this problem.
Constants I Penndc Tabln
Part A
An unknown mass of each of the following
substances, initially at 25.0°C, absorbs 1960 J of
heat, The final temperature is recorded as
indicated, Find the mass of each substance.
a. Pyrex glass (T; = 55.6 °C)
Express your answer using two significant figures.
?
m =
Submit
Previous Answers RequestAnswer
X Incorrect; Try Again; 14 attempts remaining
Part B
sand (T = 623 °C)
acer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning