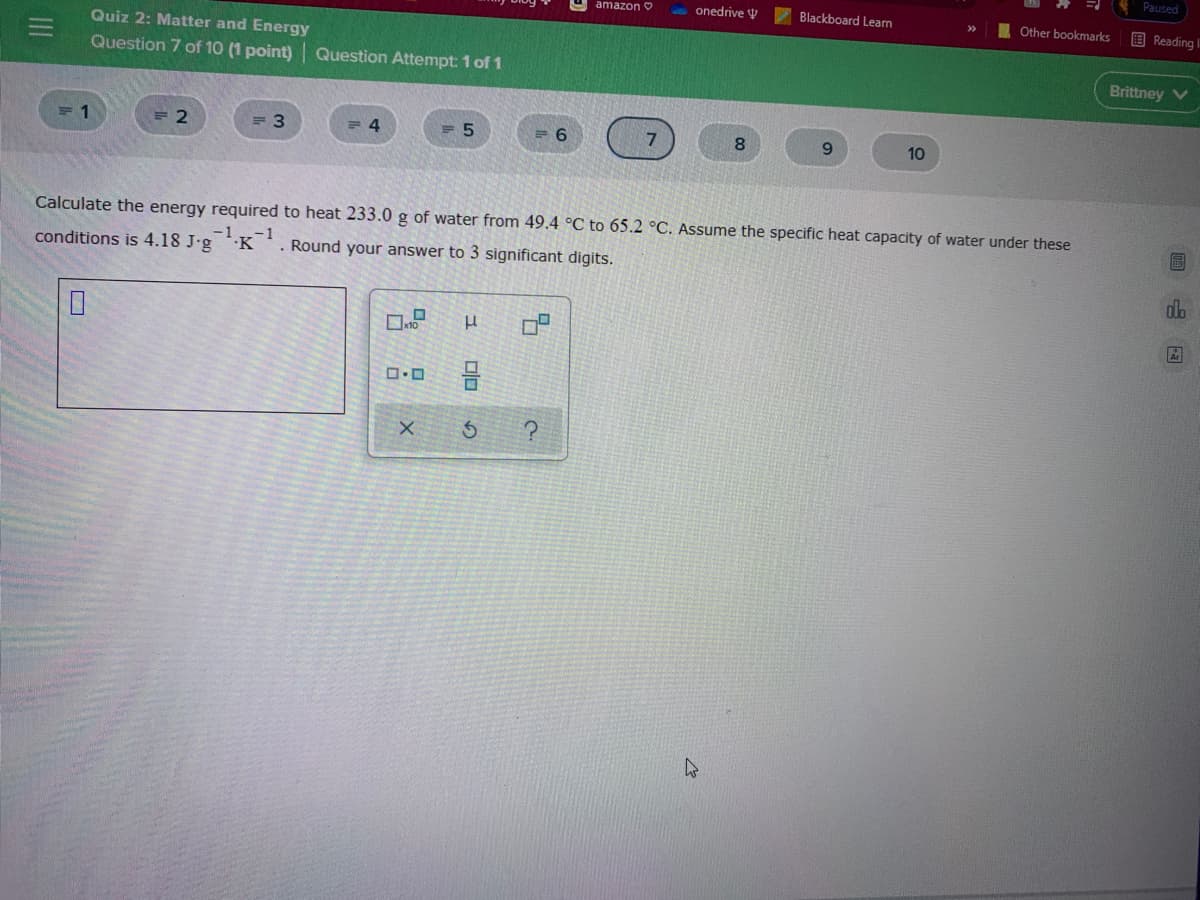
Calculate the energy required to heat 233.0 g of water from 49.4 °C to 65.2 °C. Assume the specific heat capacity of water under these conditions is 4.18 J-g -1 -1 ·K . Round your answer to 3 significant digits.
Calculate the energy required to heat 233.0 g of water from 49.4 °C to 65.2 °C. Assume the specific heat capacity of water under these conditions is 4.18 J-g -1 -1 ·K . Round your answer to 3 significant digits.
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter2: The First Law Of Thermodynamics
Section: Chapter Questions
Problem 2.12E: If the heat capacity varies withtemperature, abetter form ofequation 2.9 isto solve q=TiTfnCT-t A...
Related questions
Question
Transcribed Image Text:Paused
amazon O
onedrive V
Blackboard Learn
Other bookmarks
B Reading I
Quiz 2: Matter and Energy
Question 7 of 10 (1 point) Question Attempt: 1 of 1
Brittney V
= 2
= 3
= 4
= 5
9.
10
Calculate the energy required to heat 233.0 g of water from 49.4 °C to 65.2 °C. Assume the specific heat capacity of water under these
conditions is 4.18 J-g K'. Round your answer to 3 significant digits.
dla
II
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning