Construct a three-step synthesis of trans-2-pentene from acetylene by dragging the appropriate formulas into the bins. Note that each bin will hold only one item, and not all of the given reagents or structures will be used. (Me methyl, CHs; Et ethyl, CH3CH2.) Reactant Product Reagent 1 Reagent 2 Step 1 Product Step 2 Product Reagent 3 (acetylene) (trans-2-pentene) Li HBr На Pt 1) NaNH2 2) Me н CH3CH2NH2 H H 1) NaNH2 2) EtBr
Construct a three-step synthesis of trans-2-pentene from acetylene by dragging the appropriate formulas into the bins. Note that each bin will hold only one item, and not all of the given reagents or structures will be used. (Me methyl, CHs; Et ethyl, CH3CH2.) Reactant Product Reagent 1 Reagent 2 Step 1 Product Step 2 Product Reagent 3 (acetylene) (trans-2-pentene) Li HBr На Pt 1) NaNH2 2) Me н CH3CH2NH2 H H 1) NaNH2 2) EtBr
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter14: Elimination
Section: Chapter Questions
Problem 3E
Related questions
Question
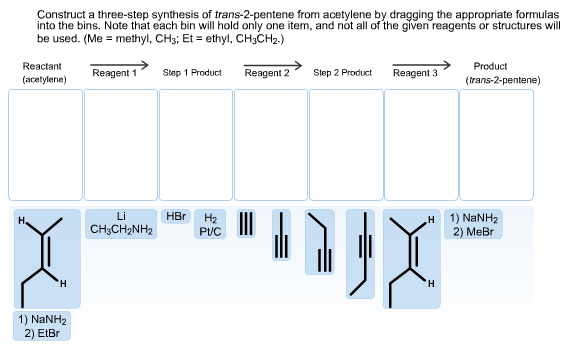
Transcribed Image Text:Construct a three-step synthesis of trans-2-pentene from acetylene by dragging the appropriate formulas
into the bins. Note that each bin will hold only one item, and not all of the given reagents or structures will
be used. (Me methyl, CHs; Et ethyl, CH3CH2.)
Reactant
Product
Reagent 1
Reagent 2
Step 1 Product
Step 2 Product
Reagent 3
(acetylene)
(trans-2-pentene)
Li
HBr
На
Pt
1) NaNH2
2) Me
н
CH3CH2NH2
H
H
1) NaNH2
2) EtBr
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning



Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

