Convergent syntheses are more efficient than linear syntheses because you risk less if one branch of your synthesis fails and because the same number of steps in a linear synthesis give a lower overall yield. For instance, in our synthesis we had three branches that converge to hexaphenylbenzene. We ran eight reactions. If the yield of each reaction was 90%, then our overall yield would be between 72 and 66 %, depending on which branch is limiting. On the other hand, if we had to do eight consecutive 90 % yield reactions, the overall yield would be 43% (0.98 x 100 %). Unfortunately it is not valid to calculate the overall yield on each branch of a convergent synthesis, only the overall yield of the branch of the limiting reagents. For instance if benzil and tetraphenylcyclopentadienone are the limiting reagents in their respective reactions, then we can only report the overall yield from benzaldehyde via benzoin. If dibenzyl ketone and tetraphenylcyclopentadienone are the limiting reagents then we can only report the overall yield from phenylacetic acid. And if diphenylacetylene is the limiting reagent then we could only report the overall yield from benzaldehyde (or benzyltriphenylphosphonium chloride if it is the limiting reagent) via stilbene. Use the following atomic weights to determine the limiting reagent branch of our synthesis. Then calculate the missing % yield(s) for that branch. Finally report the overall % yield from the starting reaction of that branch to hexaphenylbenzene. Give only 2 significant figures. If your final answer is a whole number, do not write a decimal. The grams next to an arrow are the grams of the compound previous to the arrow used in the reaction. The "grams made" are the grams of the product of that reaction. The % next to an arrow are the % yield of each respective reaction. This video through this link has further explanations of how to answer this question. C = 12, H = 1, O = 16 Answer: 85% O OH 64% 96 % + cr 56% Br 79%↓ OH 61%↓ 4.420 g 3.251 g↓ 5.172 g made 5.974 g 1.655 g↓ 4.320 g made
Convergent syntheses are more efficient than linear syntheses because you risk less if one branch of your synthesis fails and because the same number of steps in a linear synthesis give a lower overall yield. For instance, in our synthesis we had three branches that converge to hexaphenylbenzene. We ran eight reactions. If the yield of each reaction was 90%, then our overall yield would be between 72 and 66 %, depending on which branch is limiting. On the other hand, if we had to do eight consecutive 90 % yield reactions, the overall yield would be 43% (0.98 x 100 %). Unfortunately it is not valid to calculate the overall yield on each branch of a convergent synthesis, only the overall yield of the branch of the limiting reagents. For instance if benzil and tetraphenylcyclopentadienone are the limiting reagents in their respective reactions, then we can only report the overall yield from benzaldehyde via benzoin. If dibenzyl ketone and tetraphenylcyclopentadienone are the limiting reagents then we can only report the overall yield from phenylacetic acid. And if diphenylacetylene is the limiting reagent then we could only report the overall yield from benzaldehyde (or benzyltriphenylphosphonium chloride if it is the limiting reagent) via stilbene. Use the following atomic weights to determine the limiting reagent branch of our synthesis. Then calculate the missing % yield(s) for that branch. Finally report the overall % yield from the starting reaction of that branch to hexaphenylbenzene. Give only 2 significant figures. If your final answer is a whole number, do not write a decimal. The grams next to an arrow are the grams of the compound previous to the arrow used in the reaction. The "grams made" are the grams of the product of that reaction. The % next to an arrow are the % yield of each respective reaction. This video through this link has further explanations of how to answer this question. C = 12, H = 1, O = 16 Answer: 85% O OH 64% 96 % + cr 56% Br 79%↓ OH 61%↓ 4.420 g 3.251 g↓ 5.172 g made 5.974 g 1.655 g↓ 4.320 g made
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter16: Aldehydes And Ketones
Section: Chapter Questions
Problem 16.63P
Related questions
Question
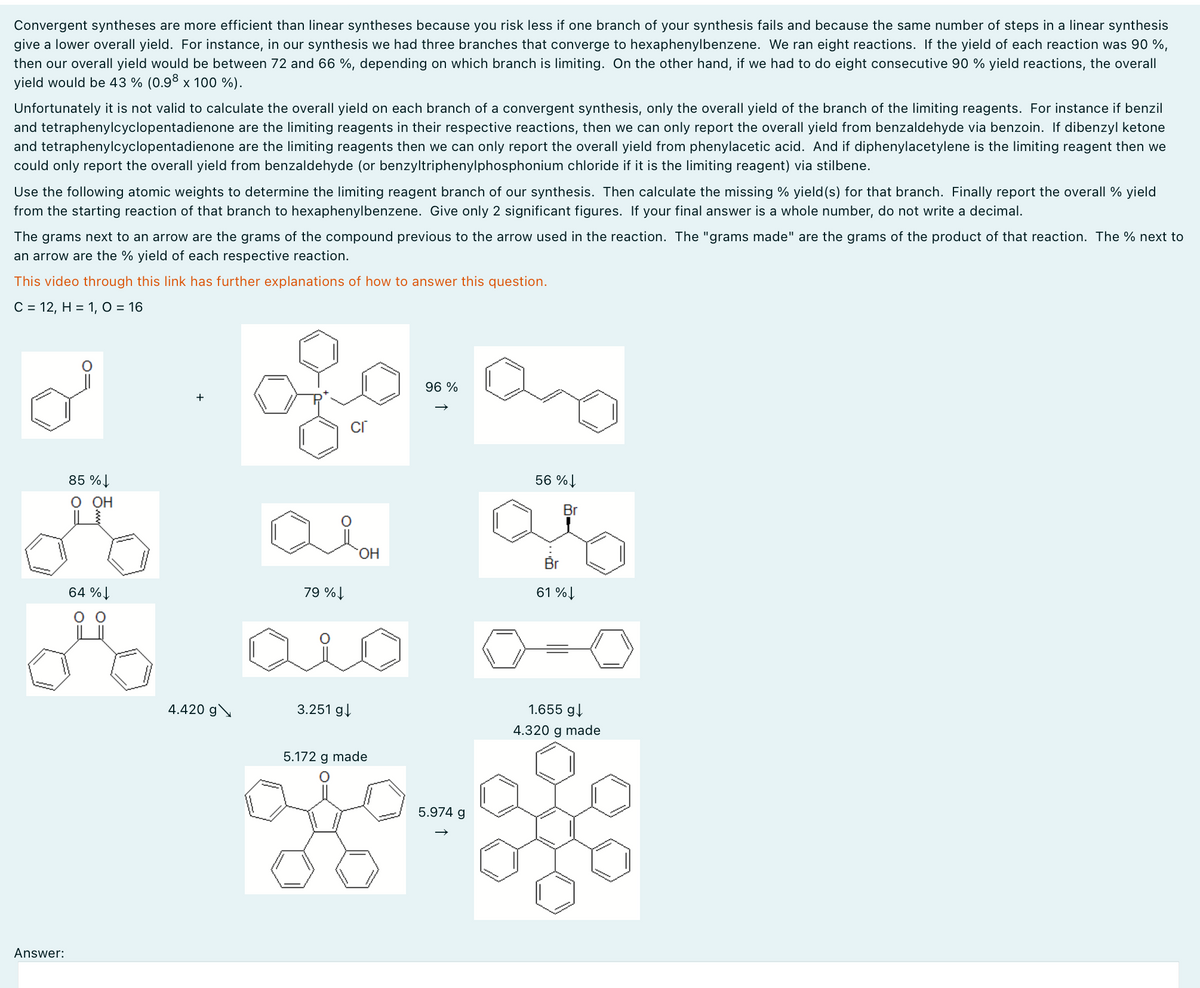
Transcribed Image Text:Convergent syntheses are more efficient than linear syntheses because you risk less if one branch of your synthesis fails and because the same number of steps in a linear synthesis
give a lower overall yield. For instance, in our synthesis we had three branches that converge to hexaphenylbenzene. We ran eight reactions. If the yield of each reaction was 90%,
then our overall yield would be between 72 and 66 %, depending on which branch is limiting. On the other hand, if we had to do eight consecutive 90 % yield reactions, the overall
yield would be 43% (0.98 x 100 %).
Unfortunately it is not valid to calculate the overall yield on each branch of a convergent synthesis, only the overall yield of the branch of the limiting reagents. For instance if benzil
and tetraphenylcyclopentadienone are the limiting reagents in their respective reactions, then we can only report the overall yield from benzaldehyde via benzoin. If dibenzyl ketone
and tetraphenylcyclopentadienone are the limiting reagents then we can only report the overall yield from phenylacetic acid. And if diphenylacetylene is the limiting reagent then we
could only report the overall yield from benzaldehyde (or benzyltriphenylphosphonium chloride if it is the limiting reagent) via stilbene.
Use the following atomic weights to determine the limiting reagent branch of our synthesis. Then calculate the missing % yield(s) for that branch. Finally report the overall % yield
from the starting reaction of that branch to hexaphenylbenzene. Give only 2 significant figures. If your final answer is a whole number, do not write a decimal.
The grams next to an arrow are the grams of the compound previous to the arrow used in the reaction. The "grams made" are the grams of the product of that reaction. The % next to
an arrow are the % yield of each respective reaction.
This video through this link has further explanations of how to answer this question.
C = 12, H = 1, O = 16
Answer:
85%
O OH
64%
96 %
+
cr
56%
Br
79%↓
OH
61%↓
4.420 g
3.251 g↓
5.172 g made
5.974 g
1.655 g↓
4.320 g made
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning
