Converting between quantities To convert from a given quantity of one reactant or product to the quantity of another reactant or product • First, convert the given quantity to moles. Use molar masses to convert masses to moles, and use Avogadro's number (6.02 x 10 particles per mole) to convert number of particles to moles. • Next, convert moles of the given reactant or product to moles of the desired reactant or product using the coefficients of the balanced chemical equation. For example, in the chemical equation2H2 + O2-2H20the coefficients tell us that 2 mol of H2 reacts with 1 mol of Oz to produce 2 mol of H20. • Finally, convert moles of the desired reactant or product back to the desired units. Again, use molar masses to convert from moles to masses, and use Avogadro's number to convert from moles to number of particles. Reaction of hydrogen and nitrogen to form ammonia Hydrogen gas, H2, reacts with nitrogen gas, N2, to form ammonia gas, NH3, according to the equation 3H2(g) + N2(g)2NH3(g) NOTE: Throughout this tutorial use molar masses expressed to five significant figures. Part A How many moles of NH3 can be produced from 12.0 mol of H2 and excess N2? Express your answer numerically in moles. • View Available Hint(s) Temglates Symbols undo redo Teset keyboard shortcuts Help mol NH3
Converting between quantities To convert from a given quantity of one reactant or product to the quantity of another reactant or product • First, convert the given quantity to moles. Use molar masses to convert masses to moles, and use Avogadro's number (6.02 x 10 particles per mole) to convert number of particles to moles. • Next, convert moles of the given reactant or product to moles of the desired reactant or product using the coefficients of the balanced chemical equation. For example, in the chemical equation2H2 + O2-2H20the coefficients tell us that 2 mol of H2 reacts with 1 mol of Oz to produce 2 mol of H20. • Finally, convert moles of the desired reactant or product back to the desired units. Again, use molar masses to convert from moles to masses, and use Avogadro's number to convert from moles to number of particles. Reaction of hydrogen and nitrogen to form ammonia Hydrogen gas, H2, reacts with nitrogen gas, N2, to form ammonia gas, NH3, according to the equation 3H2(g) + N2(g)2NH3(g) NOTE: Throughout this tutorial use molar masses expressed to five significant figures. Part A How many moles of NH3 can be produced from 12.0 mol of H2 and excess N2? Express your answer numerically in moles. • View Available Hint(s) Temglates Symbols undo redo Teset keyboard shortcuts Help mol NH3
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 41QAP: Which of the following statements is(are) true? l type='a'> A balanced equation relates the numbers...
Related questions
Question
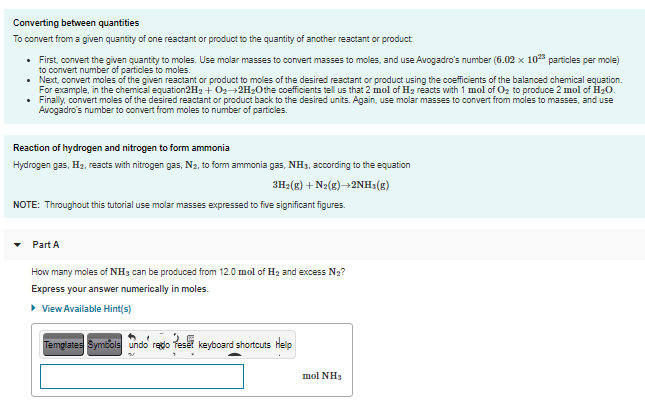
Transcribed Image Text:Converting between quantities
To convert from a given quantity of one reactant or product to the quantity of another reactant or product
• First, convert the given quantity to moles. Use molar masses to convert masses to moles, and use Avogadro's number (6.02 x 10 particles per mole)
to convert number of particles to moles.
• Next, convert moles of the given reactant or product to moles of the desired reactant or product using the coefficients of the balanced chemical equation.
For example, in the chemical equation2H2 + O2-2H20the coefficients tell us that 2 mol of H2 reacts with 1 mol of Oz to produce 2 mol of H20.
• Finally, convert moles of the desired reactant or product back to the desired units. Again, use molar masses to convert from moles to masses, and use
Avogadro's number to convert from moles to number of particles.
Reaction of hydrogen and nitrogen to form ammonia
Hydrogen gas, H2, reacts with nitrogen gas, N2, to form ammonia gas, NH3, according to the equation
3H2(g) + N2(g)2NH3(g)
NOTE: Throughout this tutorial use molar masses expressed to five significant figures.
Part A
How many moles of NH3 can be produced from 12.0 mol of H2 and excess N2?
Express your answer numerically in moles.
• View Available Hint(s)
Temglates Symbols undo redo Teset keyboard shortcuts Help
mol NH3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co