Copper(II) sulfate pentahydrate Mass of empty crucible |1.88, Mass of crucible plus hydrate 12.3 • 494 Goluration. Selt. Mass of hydrate taken Appearance of hydrate Blue Theoretical mass of H20 lost expected on heating hydrate Mass of crucible + anhydrous CuSO4 after heating. 12:30 Mass of anhydrous CuSO4. (mass of crucible + anhydrous/CUSO4 after heating – mass of empty crucible) 0:42 0•07% Mass of water lost from hydrate crystals (mass of hydrate taken – mass of anhydrous CuS04) % error in mass water loss from hydrate % of water loss Appearance of CuSO4 after heating hi ty I cluar Appearance of CUSO4 on adding water Blue.
Copper(II) sulfate pentahydrate Mass of empty crucible |1.88, Mass of crucible plus hydrate 12.3 • 494 Goluration. Selt. Mass of hydrate taken Appearance of hydrate Blue Theoretical mass of H20 lost expected on heating hydrate Mass of crucible + anhydrous CuSO4 after heating. 12:30 Mass of anhydrous CuSO4. (mass of crucible + anhydrous/CUSO4 after heating – mass of empty crucible) 0:42 0•07% Mass of water lost from hydrate crystals (mass of hydrate taken – mass of anhydrous CuS04) % error in mass water loss from hydrate % of water loss Appearance of CuSO4 after heating hi ty I cluar Appearance of CUSO4 on adding water Blue.
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.12QAP
Related questions
Question
See the attached file
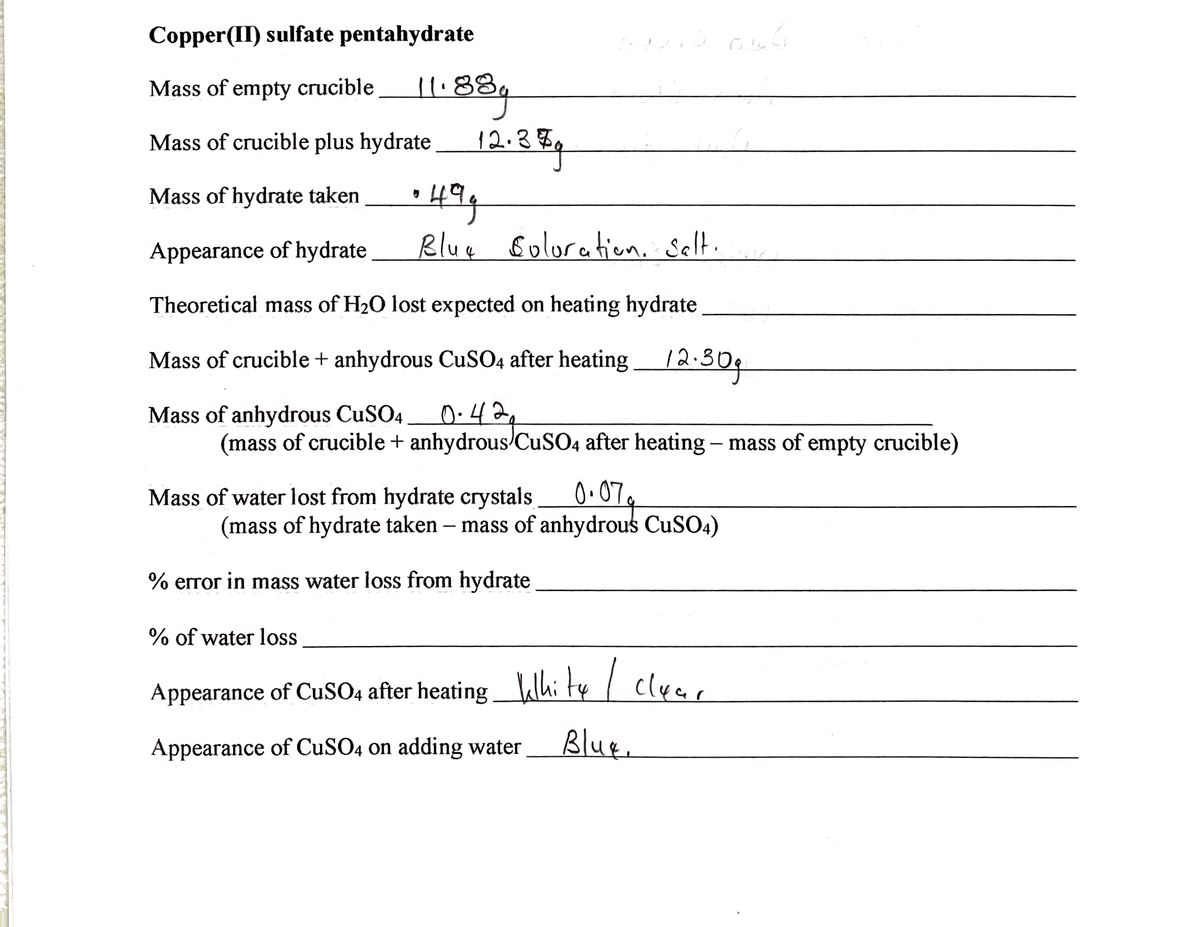
Transcribed Image Text:Copper(II) sulfate pentahydrate
Mass of empty crucible
11.88
Mass of crucible plus hydrate
12.37
Mass of hydrate taken
Appearance of hydrate
Blue Boluratien, Selt.
Theoretical mass of H20 lost expected on heating hydrate
Mass of crucible + anhydrous CuSO4 after heating
12:304
Mass of anhydrous CuSO4
(mass of crucible + anhydrous/CUSO4 after heating – mass of empty crucible)
Mass of water lost from hydrate crystals 0.07,
(mass of hydrate taken – mass of anhydrous CuS04)
% error in mass water loss from hydrate
% of water loss
Appearance of CuSO4 after heatingWhi ty/ clya,
Appearance of CuSO4 on adding water
Blue.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning