1. Formula of anhydrous salt ........ ........ 2. Mass of empty dry crucible and lid 19.219.8 3. Mass of crucible, lid and the hydrate before heating 20.432 S. 4. Mass of crucible, lid and residue (anhydrous salt) after heating 2.0. 211 5, Mass oferucible, Ind and residue (abhydrous salt) aftecadditiona-heating 6. Mass of hydrate, (#3 – #2) ...... ......... 7. Mass of residue, the anhydrous salt ((smaller of #5 or #4) – #2) ...... ......... 8. Mass of water lost, (#6 – #7) ...... 9. Molar mass of water .... 10. Molar mass of anhydrous salt ... ....... mass of water lost, # 8 11. Moles of water= molar mass of water, #9
1. Formula of anhydrous salt ........ ........ 2. Mass of empty dry crucible and lid 19.219.8 3. Mass of crucible, lid and the hydrate before heating 20.432 S. 4. Mass of crucible, lid and residue (anhydrous salt) after heating 2.0. 211 5, Mass oferucible, Ind and residue (abhydrous salt) aftecadditiona-heating 6. Mass of hydrate, (#3 – #2) ...... ......... 7. Mass of residue, the anhydrous salt ((smaller of #5 or #4) – #2) ...... ......... 8. Mass of water lost, (#6 – #7) ...... 9. Molar mass of water .... 10. Molar mass of anhydrous salt ... ....... mass of water lost, # 8 11. Moles of water= molar mass of water, #9
Chemical Principles in the Laboratory
11th Edition
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Chapter6: Properties Of Hydrates
Section: Chapter Questions
Problem 1ASA: A student is given a sample of a pink manganese (II) chloride hydrate. She weighs the sample in a...
Related questions
Question
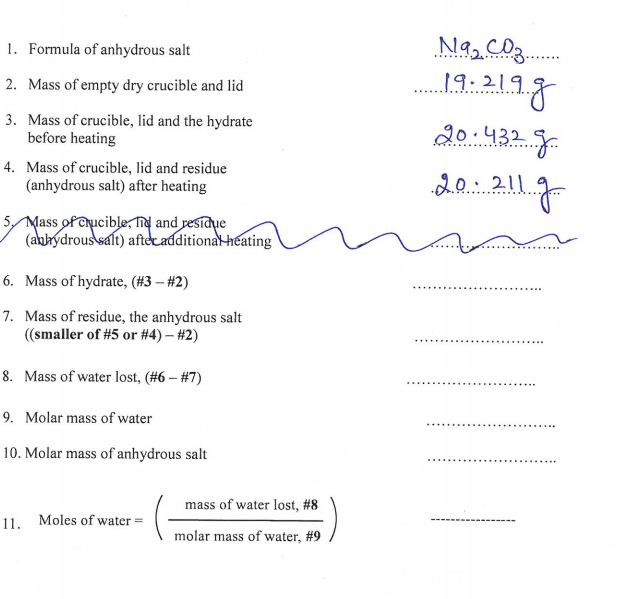
Transcribed Image Text:1. Formula of anhydrous salt
19:211g
2. Mass of empty dry crucible and lid
3. Mass of crucible, lid and the hydrate
before heating
20.432.8.
4. Mass of crucible, lid and residue
(anhydrous salt) after heating
2.0.211
5 Mass of Crucible, nd and residuụe
(abhydrous salt) aftecadditional heating
6. Mass of hydrate, (#3 – #2)
7. Mass of residue, the anhydrous salt
((smaller of #5 or #4) – #2)
8. Mass of water lost, (#6 – #7)
9. Molar mass of water
10. Molar mass of anhydrous salt
mass of water lost, #8
11. Moles of water
molar mass of water, #9
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole