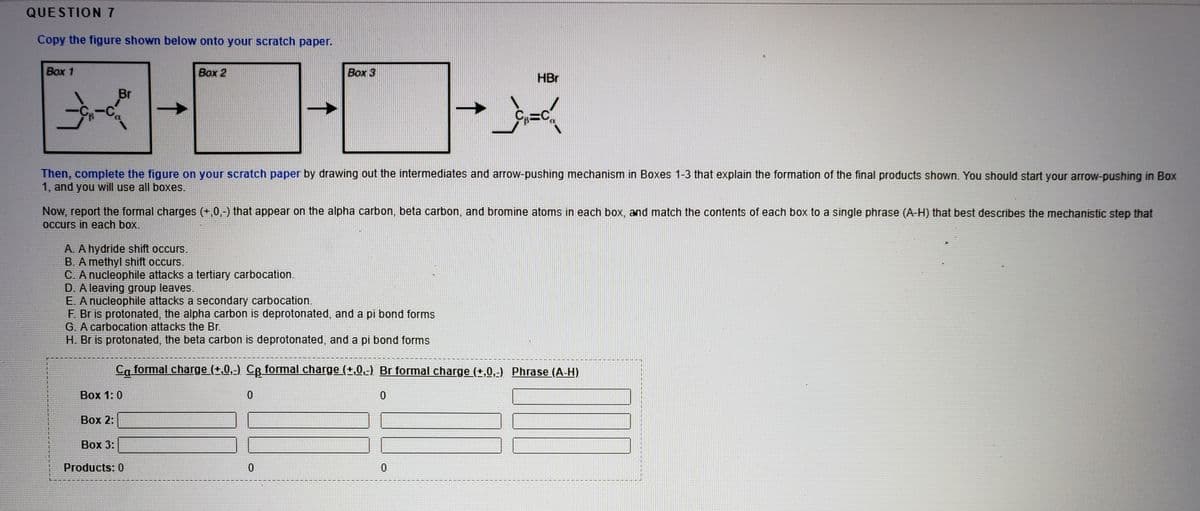
Copy the figure shown below onto your scratch paper. Box 1 Вох 2 Box 3 HBr Br Then, complete the figure on your scratch paper by drawing out the intermediates and arrow-pushing mechanism in Boxes 1-3 that explain the formation of the final products shown. You should start your arrow-pushing in Box 1, and you will use all boxes. Now, report formal charges (+,0,-) that appear on the alpha carbon, beta carbon, and bromine atoms in each box, and match the contents of each box to a single phrase (A-H) that best describes the mechanistic step that occurs in each box. A. A hydride shift occurs. B. A methyl shift occurs. C. A nucleophile attacks a tertiary carbocation. D. A leaving group leaves. E. A nucleophile attacks a secondary carbocation. F. Br is protonated, the alpha carbon is deprotonated, and a pi bond forms G. A carbocation attacks the Br. H. Br is protonated, the beta carbon is deprotonated, and a pi bond forms Ca formal charge (+.0,-) Ce formal charge (+,0.-) Br formal charge (+,0.-) Phrase (A-H) Box 1: 0 Box 2: Вох 3: Products: 0
Catalysis and Enzymatic Reactions
Catalysis is the kind of chemical reaction in which the rate (speed) of a reaction is enhanced by the catalyst which is not consumed during the process of reaction and afterward it is removed when the catalyst is not used to make up the impurity in the product. The enzymatic reaction is the reaction that is catalyzed via enzymes.
Lock And Key Model
The lock-and-key model is used to describe the catalytic enzyme activity, based on the interaction between enzyme and substrate. This model considers the lock as an enzyme and the key as a substrate to explain this model. The concept of how a unique distinct key only can have the access to open a particular lock resembles how the specific substrate can only fit into the particular active site of the enzyme. This is significant in understanding the intermolecular interaction between proteins and plays a vital role in drug interaction.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images




