Course Home +. ge.com/course.html?courseld=15807691&0penVellumHMAC=1d3e42816b27b369fba593da0106e31d#10001 View Available Hint(s) HẢ Units Value P Pearson Contact Us Privacy Policy I Permissions Terms of Use Copyright O 2020 Pearson Education Inc. All rights reserved. 1:16 A 2/14/20 34% Insert PrtSc F12 F11 F10 F9 F8 F7 F6 F5 F4 F3 F2 %$4 9- 8 + II 23
Course Home +. ge.com/course.html?courseld=15807691&0penVellumHMAC=1d3e42816b27b369fba593da0106e31d#10001 View Available Hint(s) HẢ Units Value P Pearson Contact Us Privacy Policy I Permissions Terms of Use Copyright O 2020 Pearson Education Inc. All rights reserved. 1:16 A 2/14/20 34% Insert PrtSc F12 F11 F10 F9 F8 F7 F6 F5 F4 F3 F2 %$4 9- 8 + II 23
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
Transcribed Image Text:Course Home
+.
ge.com/course.html?courseld=15807691&0penVellumHMAC=1d3e42816b27b369fba593da0106e31d#10001
<Chapter 3
Item 13
13 of
I Review | Constants
Periodic
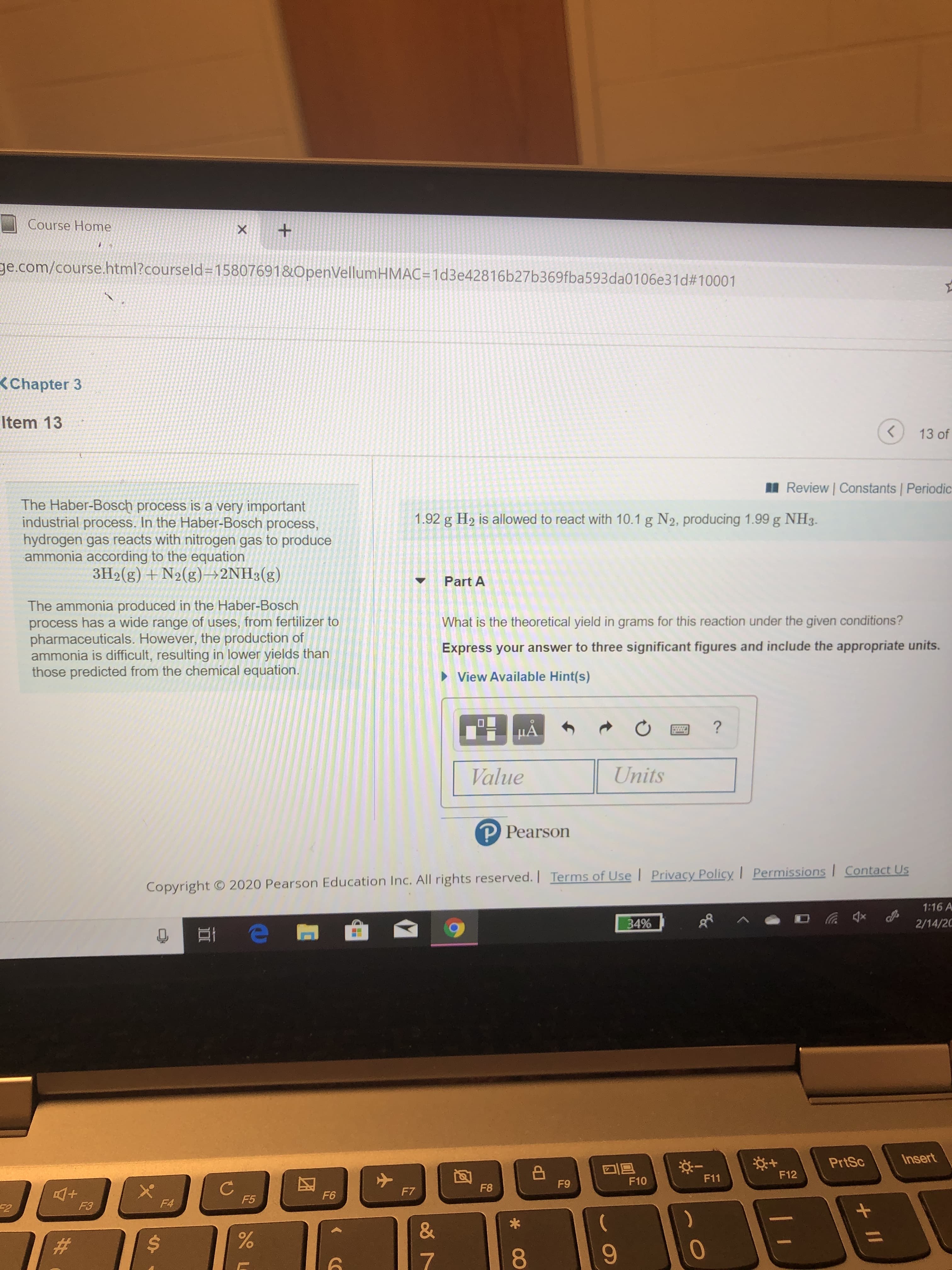
The Haber-Bosch process is a very important
industrial process. In the Haber-Bosch process,
hydrogen gas reacts with nitrogen gas to produce
ammonia according to the equation
3H2(g) +N2(g)→2NH3(g)
1.92 g H2 is allowed to react with 10.1 g N2, producing 1.99 g NH3.
Part A
The ammonia produced in the Haber-Bosch
process has a wide range of uses, from fertilizer to
pharmaceuticals. However, the production of
ammonia is difficult, resulting in lower yields than
those predicted from the chemical equation.
What is the theoretical yield in grams for this reaction under the given conditions?
Express your answer to three significant figures and include the appropriate units.
> View Available Hint(s)
HẢ
Units
Value
P Pearson
Contact Us
Privacy Policy I Permissions
Terms of Use
Copyright O 2020 Pearson Education Inc. All rights reserved.
1:16 A
2/14/20
34%
Insert
PrtSc
F12
F11
F10
F9
F8
F7
F6
F5
F4
F3
F2
%$4
9-
8
+ II
23
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY