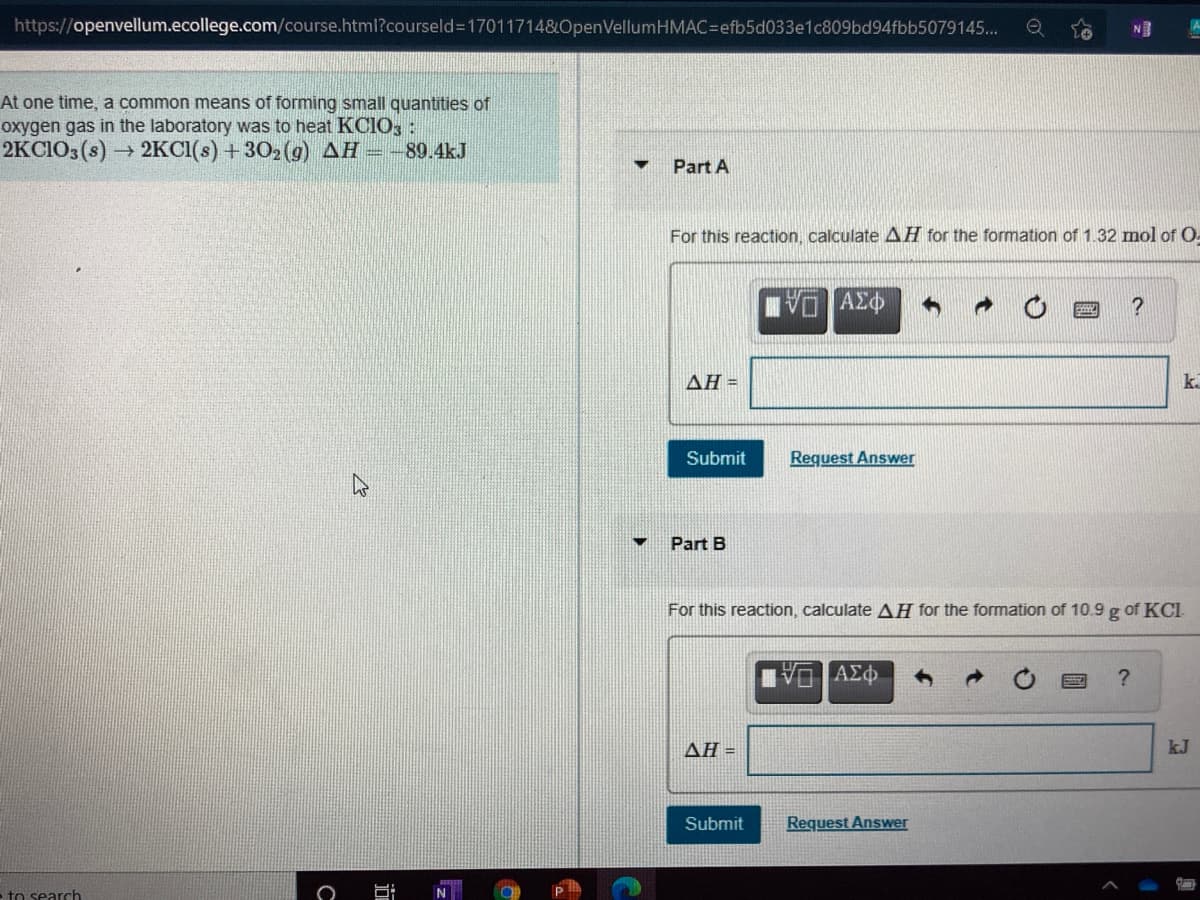
https://openvellum.ecollege.com/course.html?courseld3170117148&OpenVellumHMAC3Defb5d033e1c809bd94fbb5079145... t one time, a common means of forming small quantities of xygen gas in the laboratory was to heat KCIO, : EKCIO3(s) 2KCI(s) +302(g) AH =-89.4kJ Part A For this reaction, calculate AH for the formation of 1.32 mol of O- ΑΣφ ? ΔΗ- k. Submit Request Answer Part B For this reaction, calculate AH for the formation of 10.9 g of KCI ? ΔΗ- kJ Submit Request Answer
https://openvellum.ecollege.com/course.html?courseld3170117148&OpenVellumHMAC3Defb5d033e1c809bd94fbb5079145... t one time, a common means of forming small quantities of xygen gas in the laboratory was to heat KCIO, : EKCIO3(s) 2KCI(s) +302(g) AH =-89.4kJ Part A For this reaction, calculate AH for the formation of 1.32 mol of O- ΑΣφ ? ΔΗ- k. Submit Request Answer Part B For this reaction, calculate AH for the formation of 10.9 g of KCI ? ΔΗ- kJ Submit Request Answer
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter8: Thermochemistry
Section: Chapter Questions
Problem 70QAP: Consider the reaction between methane and oxygen producing carbon dioxide and water. Suppose that...
Related questions
Question
Plz help and show work plz be quick
Transcribed Image Text:https://openvellum.ecollege.com/course.html?courseld317011714&OpenVellumHMAC=efb5d033e1c809bd94fbb5079145...
NE
At one time, a common means of forming small quantities of
oxygen gas in the laboratory was to heat KCIO, :
2KCIO3(s) 2KCI(s) +302 (g) AH --89.4kJ
Part A
For this reaction, calculate AH for the formation of 1.32 mol of O-
ΔΗ-
k.
Submit
Request Answer
Part B
For this reaction, calculate AH for the formation of 10.9 g of KCI
?
ΔΗ-
kJ
Submit
Request Answer
-to search
近
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning