Chapter84: Fractional Distillation, Azeotropes
Section: Chapter Questions
Problem 7P
Related questions
Question
create a schematic diagram for the provided procedure
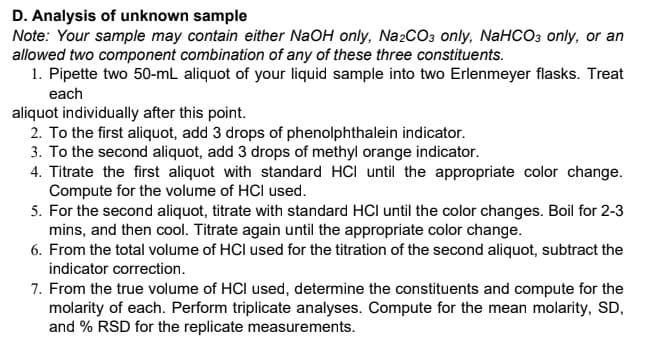
Transcribed Image Text:D. Analysis of unknown sample
Note: Your sample may contain either NaOH only, Na2CO3 only, NaHCO3 only, or an
allowed two component combination of any of these three constituents.
1. Pipette two 50-mL aliquot of your liquid sample into two Erlenmeyer flasks. Treat
each
aliquot individually after this point.
2. To the first aliquot, add 3 drops of phenolphthalein indicator.
3. To the second aliquot, add 3 drops of methyl orange indicator.
4. Titrate the first aliquot with standard HCI until the appropriate color change.
Compute for the volume of HCl used.
5. For the second aliquot, titrate with standard HCl until the color changes. Boil for 2-3
mins, and then cool. Titrate again until the appropriate color change.
6. From the total volume of HCl used for the titration of the second aliquot, subtract the
indicator correction.
7. From the true volume of HCI used, determine the constituents and compute for the
molarity of each. Perform triplicate analyses. Compute for the mean molarity, SD,
and % RSD for the replicate measurements.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT



EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

