Current Attempt in Progress What is the coefficient of H2O when the following equation is properly balanced with the smallest set of whole numbers? Na + H2O →_ NaOH+_H2 O 1 O 5 0 4 O 2 eTextbook and Media Save for Later Attempts: 0 of 2 used Submit A o search N 99+
Current Attempt in Progress What is the coefficient of H2O when the following equation is properly balanced with the smallest set of whole numbers? Na + H2O →_ NaOH+_H2 O 1 O 5 0 4 O 2 eTextbook and Media Save for Later Attempts: 0 of 2 used Submit A o search N 99+
Chapter3: Statistical Tests With Excel
Section: Chapter Questions
Problem 7P
Related questions
Question
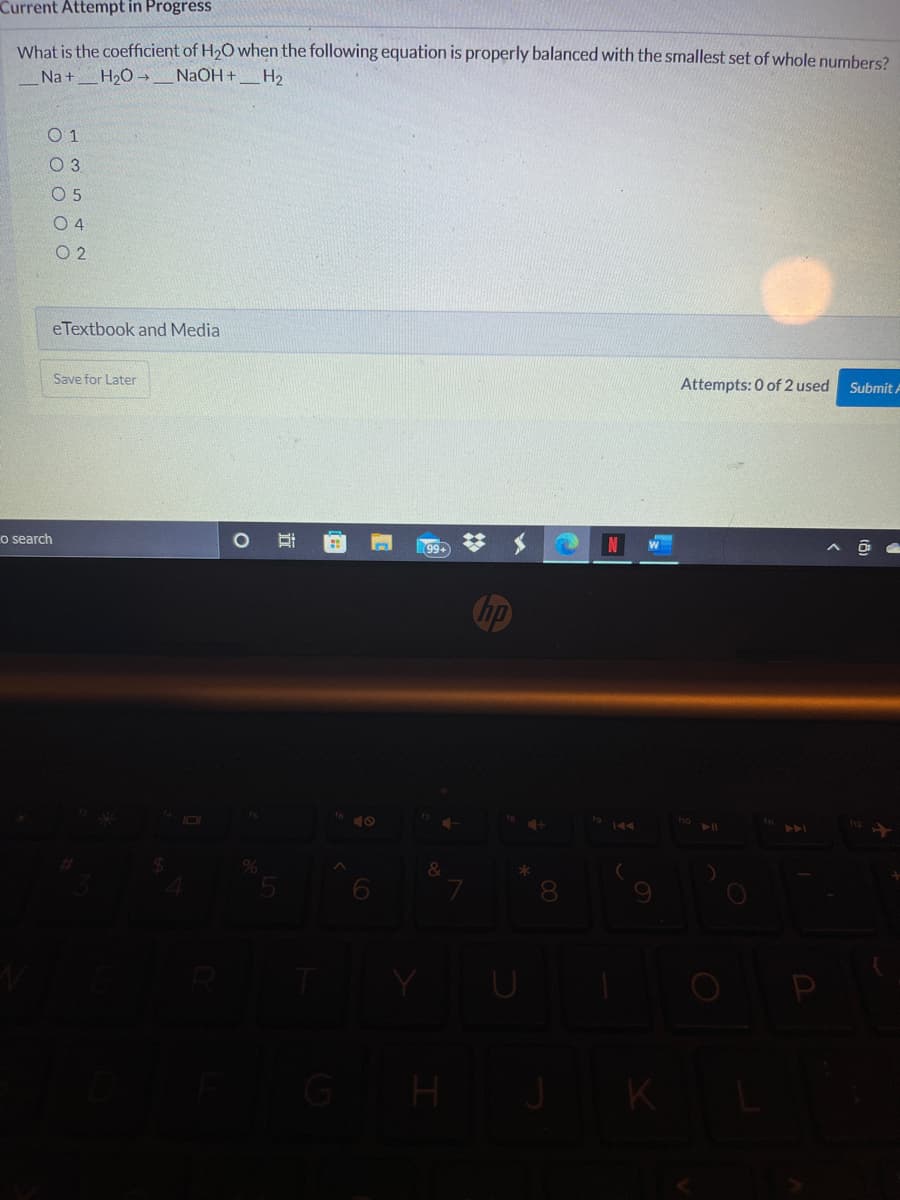
Transcribed Image Text:Current Attempt in Progress
What is the coefficient of H2O when the following equation is properly balanced with the smallest set of whole numbers?
Na +H2O -
NaOH +_H2
0 1
O 5
O 4
O 2
eTextbook and Media
Save for Later
Attempts: 0 of 2 used
Submit A
o search
99+
近
Expert Solution
Step 1
Balanced equation is the equation in which equal number of atoms of every element is present on both reactant and product side.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

