lasks attempted The following two-step process has a 60.0 % yield for each step. CH4 + 4 Cl2 –→ CCl, + 4 HC1 | CCl, + 2 HF –→ CCl,F2 + 2 HCi The CCl, that is formed in the first step of the process is used as the reactant for the second step. Assuming 6.00 mol of CH, are used in the reaction with excess amounts of both Cl, and HF, how many total moles of HCl would be formed? HCI mol SAVE RESPONSE 8 10 18
lasks attempted The following two-step process has a 60.0 % yield for each step. CH4 + 4 Cl2 –→ CCl, + 4 HC1 | CCl, + 2 HF –→ CCl,F2 + 2 HCi The CCl, that is formed in the first step of the process is used as the reactant for the second step. Assuming 6.00 mol of CH, are used in the reaction with excess amounts of both Cl, and HF, how many total moles of HCl would be formed? HCI mol SAVE RESPONSE 8 10 18
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter4: Chemical Reactions
Section: Chapter Questions
Problem 4.97P
Related questions
Question
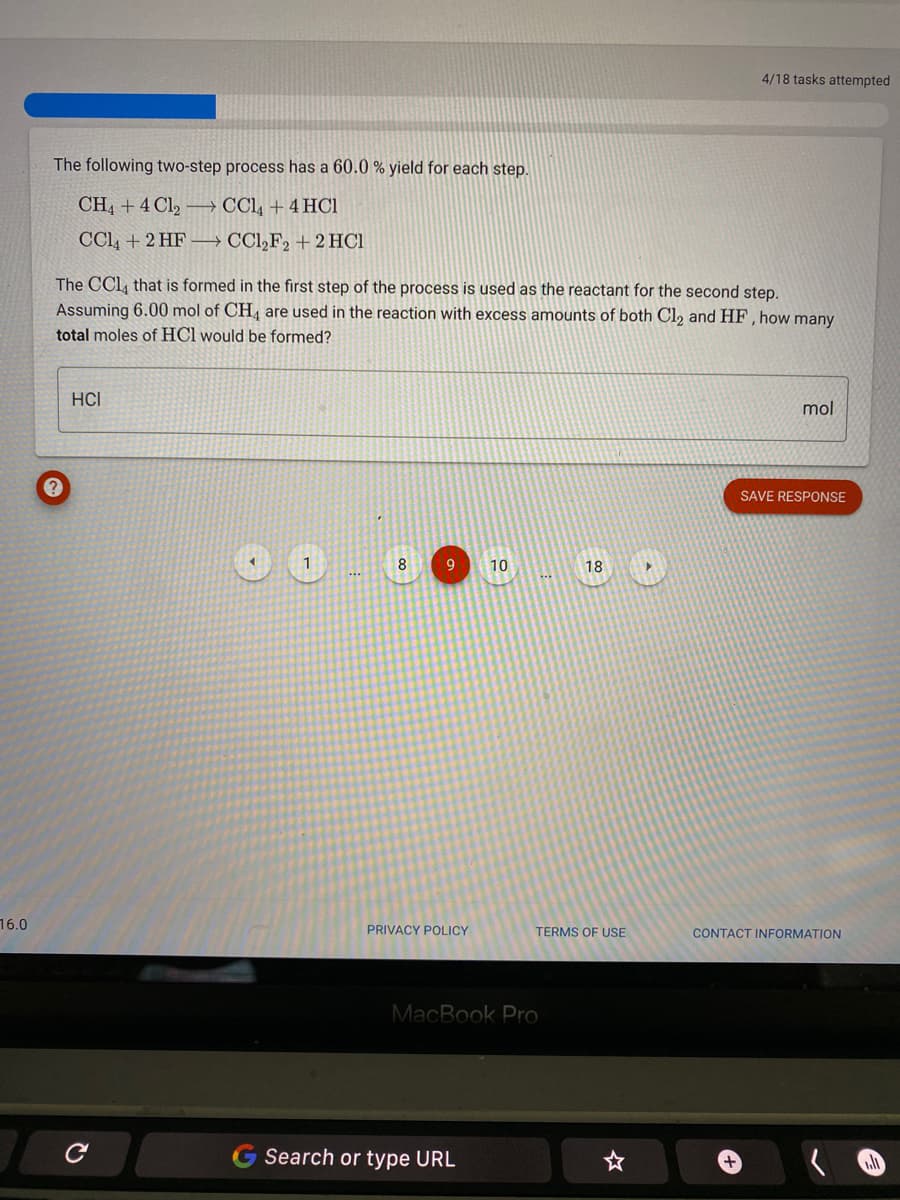
Transcribed Image Text:4/18 tasks attempted
The following two-step process has a 60.0 % yield for each step.
CH4 + 4 Cl, → CCl, + 4 HC1
CCl, + 2 HF → CCl,F2 + 2 HC1
The CCl, that is formed in the first step of the process is used as the reactant for the second step.
Assuming 6.00 mol of CH, are used in the reaction with excess amounts of both Cl, and HF, how many
total moles of HCl would be formed?
HCI
mol
SAVE RESPONSE
1
8
10
18
16.0
PRIVACY POLICY
TERMS OF USE
CONTACT INFORMATION
MacBook Pro
G Search or type URL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning