Q Search this co References Use the References to access important values if needed for this question. According to the following reaction, how many grams of chlorine gas are required for the complete reaction of 26.3 grams of phosphorus (P)? Phosphorus (P,)(s) + chlorine(g) > phosphorus trichloride(l) grams chlorine gas Submit Answer : This is group attempt 1 of 5 Stolchiometry of Roactions: Gra... Next Beck FED tv
Q Search this co References Use the References to access important values if needed for this question. According to the following reaction, how many grams of chlorine gas are required for the complete reaction of 26.3 grams of phosphorus (P)? Phosphorus (P,)(s) + chlorine(g) > phosphorus trichloride(l) grams chlorine gas Submit Answer : This is group attempt 1 of 5 Stolchiometry of Roactions: Gra... Next Beck FED tv
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 62QAP: An air bag is deployed by utilizing the following re tion the nitrogen gas produced inflates the air...
Related questions
Question
100%
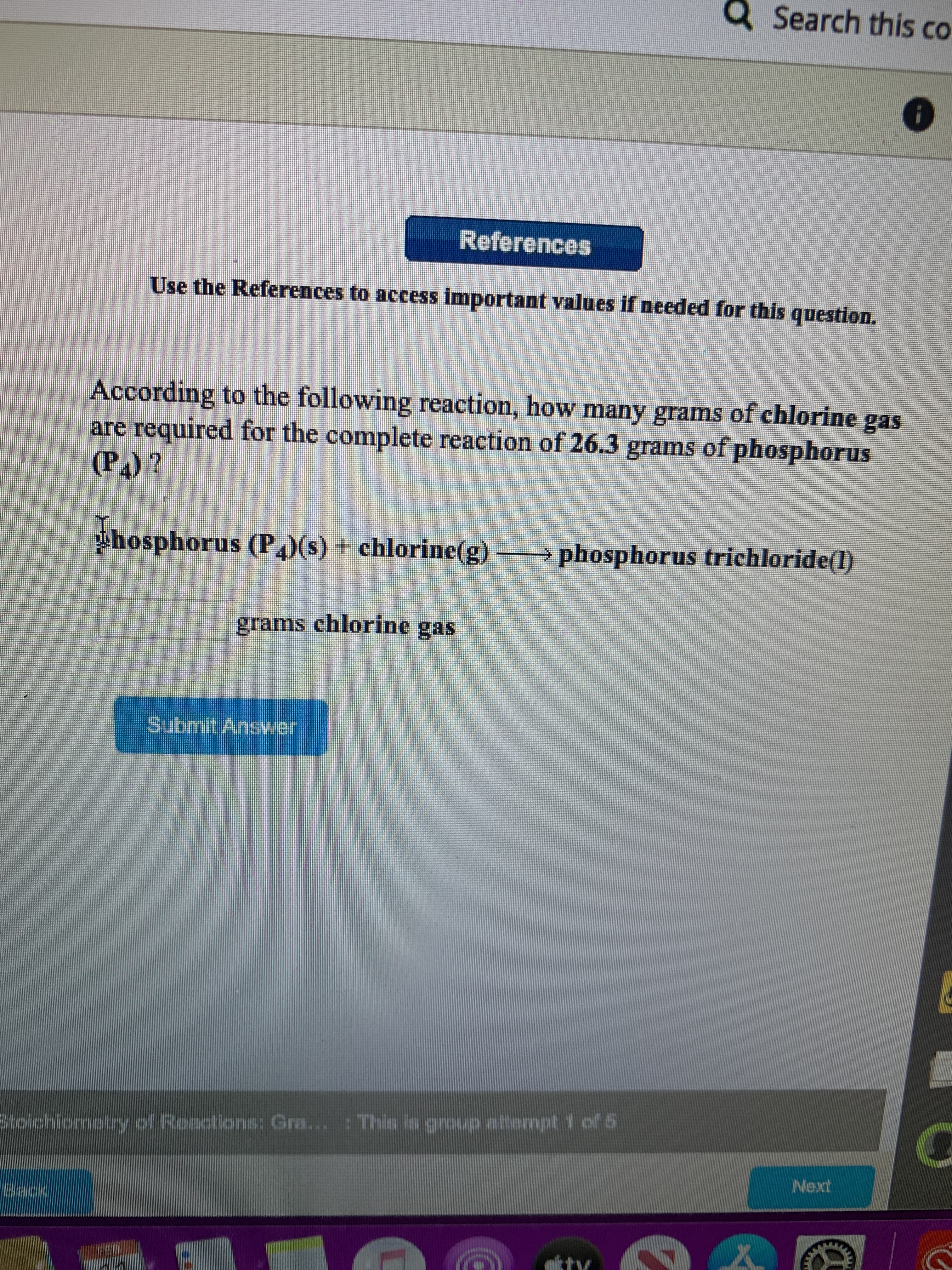
Transcribed Image Text:Q Search this co
References
Use the References to access important values if needed for this question.
According to the following reaction, how many grams of chlorine gas
are required for the complete reaction of 26.3 grams of phosphorus
(P)?
Phosphorus (P,)(s) + chlorine(g)
> phosphorus trichloride(l)
grams chlorine gas
Submit Answer
: This is group attempt 1 of 5
Stolchiometry of Roactions: Gra...
Next
Beck
FED
tv
Expert Solution
Step 1
The number of moles for a given chemical species refers to the ratio of mass of the species to that of its molar mass. The formula is shown below:
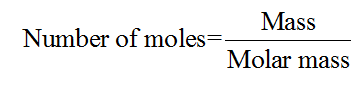
Step 2
In the given problem, the reaction is as follows:
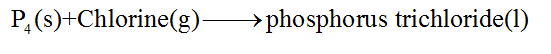
Step 3
The balanced chemical equation for the given reaction is as follows:
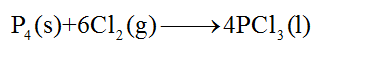
Step 4
The mass of phosphorus is given to be 26.3 g.
From the balanced chemical equation, it is clear that 1 mole of phosphorus requires 6 moles of chlorine gas. The number of moles of phosphorus present in 26.3 grams of phosphorus can be calculated as follows:
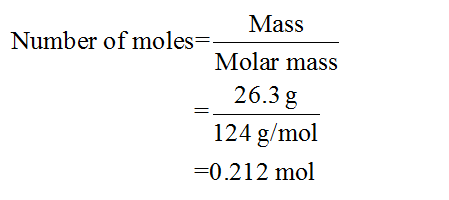
Step by step
Solved in 7 steps with 6 images

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div