(d) Complete the following statements: (1) The three are solid, liquid and (i) The three scales are degree Celsius, Kelvin and (iii) Pure substances have a composition whereas mixtures have a composition. Homogeneous mixtures have a composition. When a solute
(d) Complete the following statements: (1) The three are solid, liquid and (i) The three scales are degree Celsius, Kelvin and (iii) Pure substances have a composition whereas mixtures have a composition. Homogeneous mixtures have a composition. When a solute
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter1: Matter And Measurements
Section: Chapter Questions
Problem 72QAP: Given the following solubility curves, answer the following questions: (a) In which of the two...
Related questions
Question
100%
What are the answers in the gaps?
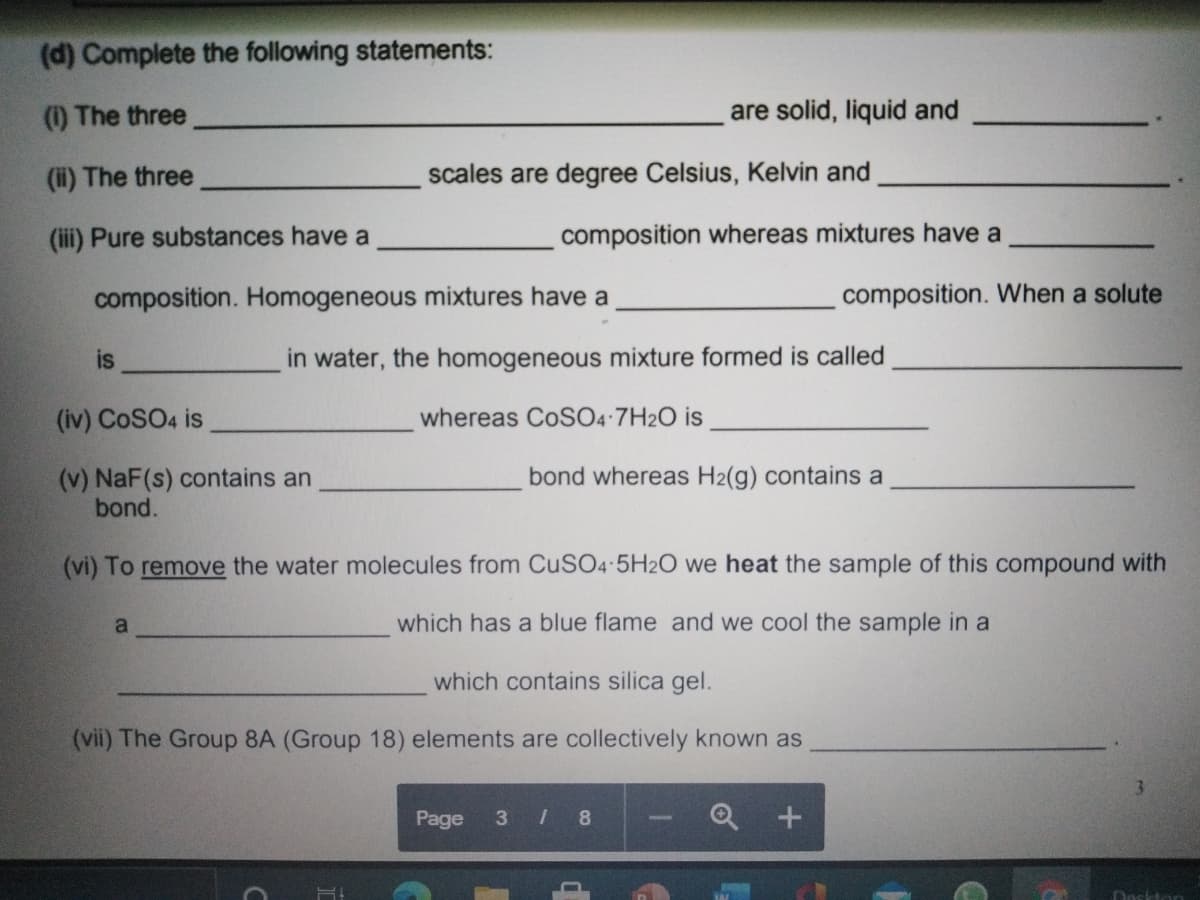
Transcribed Image Text:(d) Complete the following statements:
(1) The three
are solid, liquid and
(i) The three
scales are degree Celsius, Kelvin and
(ii) Pure substances have a
composition whereas mixtures have a
composition. Homogeneous mixtures have a
composition. When a solute
is
in water, the homogeneous mixture formed is called
(iv) CoSO4 is
whereas CoSO4 7H2O is
(v) NaF(s) contains an
bond.
bond whereas H2(g) contains a
(vi) To remove the water molecules from CuSO4 5H2O we heat the sample of this compound with
a
which has a blue flame and we cool the sample in a
which contains silica gel.
(vii) The Group 8A (Group 18) elements are collectively known as
3.
Page
3 / 8
Dosktan
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning