d) For a reaction where the equilibrium constant is greater than the reaction quotient, as the reaction restores equilibrium the concentration of products will decrease. e) One assumption of kinetic molecular theory is that collisions are elastic, meaning that gas particles do not have intermolecular forces.
d) For a reaction where the equilibrium constant is greater than the reaction quotient, as the reaction restores equilibrium the concentration of products will decrease. e) One assumption of kinetic molecular theory is that collisions are elastic, meaning that gas particles do not have intermolecular forces.
Living By Chemistry: First Edition Textbook
1st Edition
ISBN:9781559539418
Author:Angelica Stacy
Publisher:Angelica Stacy
ChapterU6: Showtime: Reversible Reactions And Chemical Equilibrium
Section: Chapter Questions
Problem SII3RE
Related questions
Question
d and e only please
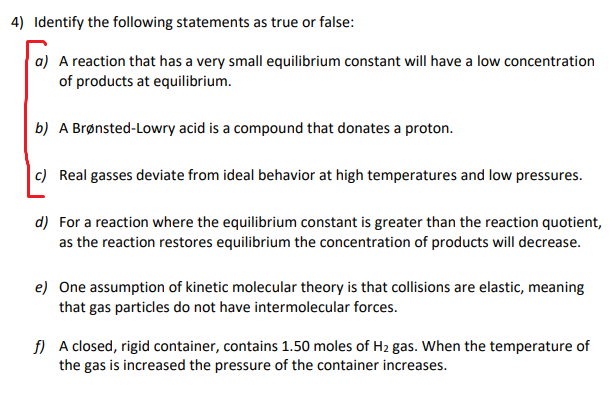
Transcribed Image Text:4) Identify the following statements as true or false:
a) A reaction that has a very small equilibrium constant will have a low concentration
of products at equilibrium.
b) A Brønsted-Lowry acid is a compound that donates a proton.
c) Real gasses deviate from ideal behavior at high temperatures and low pressures.
d) For a reaction where the equilibrium constant is greater than the reaction quotient,
as the reaction restores equilibrium the concentration of products will decrease.
e) One assumption of kinetic molecular theory is that collisions are elastic, meaning
that gas particles do not have intermolecular forces.
f) A closed, rigid container, contains 1.50 moles of H2 gas. When the temperature of
the gas is increased the pressure of the container increases.
Expert Solution
Step 1
Welcome to bartleby !
Step 2
Explanation :
D)Statement is false ,if K>Q then in that case reactant will convert into products .If K<Q would have given in the question in that products concentration will decrease .
E)True statement ,because postulates of kinetic theory consider only ideal gas molecules and in ideal gas molecules we don't have intermolecular force of attraction .
Also elastic collision means molecules don't have any attraction .
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning