Endothermic reaction Exothermic reaction E(forward) > E,(reverse) E,(reverse) > E,(forward) E,(reverse) E,(forward) E,(forward) E,(reverse) ΔΕ > 0 AE < 0 A Figure 15.15 The energy profile for an endothermic reaction (left) and an exothermic reaction (right).
Endothermic reaction Exothermic reaction E(forward) > E,(reverse) E,(reverse) > E,(forward) E,(reverse) E,(forward) E,(forward) E,(reverse) ΔΕ > 0 AE < 0 A Figure 15.15 The energy profile for an endothermic reaction (left) and an exothermic reaction (right).
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter4: Polar Bonds, Polar Reactions
Section: Chapter Questions
Problem 29CTQ
Related questions
Question
Consider the equilibrium A ⇌ B in which both the
forward and reverse reactions are elementary (single-step)
reactions. Assume that the only effect of a catalyst on the
reaction is to lower the activation energies of the forward
and reverse reactions. Using the
Arrhenius equation, prove that the equilibrium
constant is the same for the catalyzed reaction as for
the uncatalyzed one.
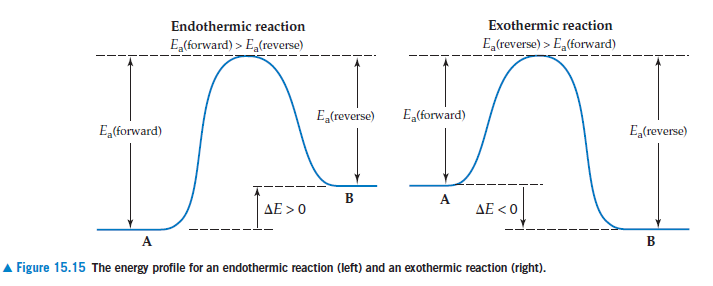
Transcribed Image Text:Endothermic reaction
Exothermic reaction
E(forward) > E,(reverse)
E,(reverse) > E,(forward)
E,(reverse)
E,(forward)
E,(forward)
E,(reverse)
ΔΕ > 0
AE < 0
A
Figure 15.15 The energy profile for an endothermic reaction (left) and an exothermic reaction (right).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 8 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning