D STARTING AMOUNT X Your Aktiv Learning trial expires on 09/05/22 at 11:59 PM Question 28 of 29 What is the molality of lithium ions in a 0.302 m solution of Li,PO, assuming the compound dissociates completely? mol Li 6.022 x 10 0.001 35.0 mol Li,PO. Li,PO. 115.79 0.302 m Li m Li,PO. 3 0.906 1.21 0.101 g solvent kg solvent 1000 QU' Activate Now Submit
D STARTING AMOUNT X Your Aktiv Learning trial expires on 09/05/22 at 11:59 PM Question 28 of 29 What is the molality of lithium ions in a 0.302 m solution of Li,PO, assuming the compound dissociates completely? mol Li 6.022 x 10 0.001 35.0 mol Li,PO. Li,PO. 115.79 0.302 m Li m Li,PO. 3 0.906 1.21 0.101 g solvent kg solvent 1000 QU' Activate Now Submit
Chapter13: Titrations In Analytical Chemistry
Section: Chapter Questions
Problem 13.12QAP
Related questions
Question
Chemistry
pls help, I dont understand how this software wants me to enter the answer. its asking lfr the starting amount but its not taking it in the way Im putting it
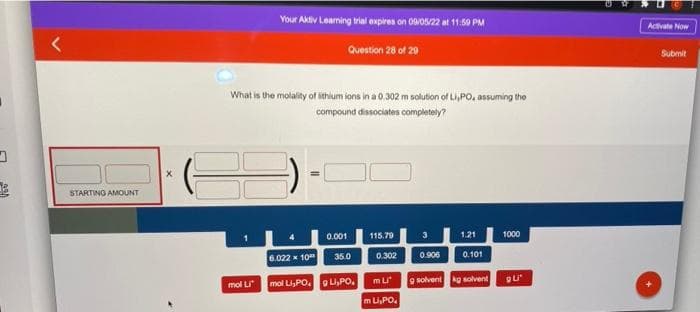
Transcribed Image Text:D
STARTING AMOUNT
X
Your Aktiv Learning trial expires on 09/05/22 at 11:59 PM
Question 28 of 29
What is the molality of lithium ions in a 0.302 m solution of Li,PO, assuming the
compound dissociates completely?
mol Li
6.022 x 10
0.001
35.0
mol Li,POL,PO.
115.79
0.302
m Li
m Li,PO
3
0.906
1.21
0.101
9 solvent kg solvent
1000
QU'
Activate Now
Submit
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning