Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter23: Organic Polymers, Natural And Synthetic
Section: Chapter Questions
Problem 47QAP: Plants synthesize carbohydrates from CO2 and H2O by the process of photosynthesis. For example,...
Related questions
Question
Need part d in this problem
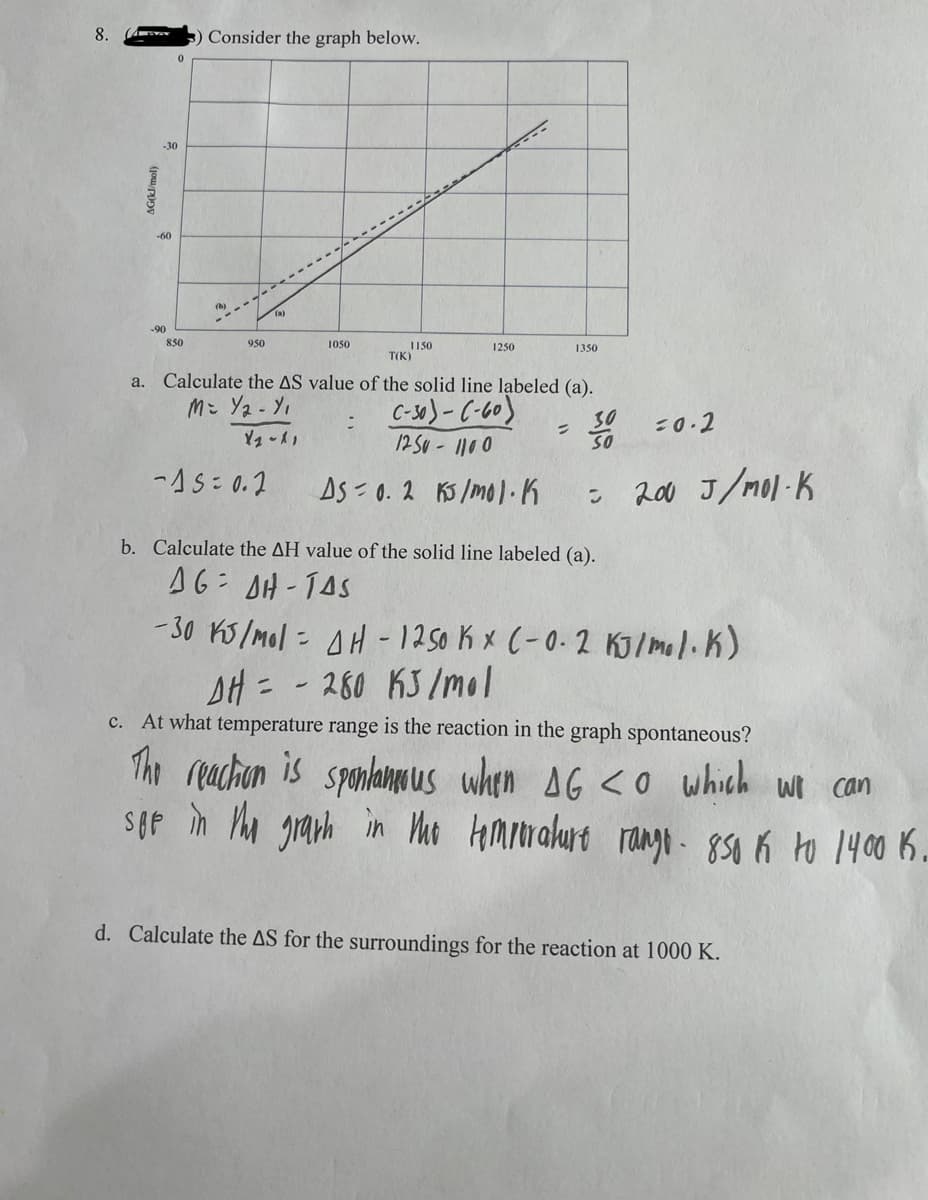
Transcribed Image Text:8.
AG(kJ/mol)
a.
3) Consider the graph below.
0
-30
-60
850
(b)
950
1050
-15= 0.2
-----
:
1150
1250
T(K)
Calculate the AS value of the solid line labeled (a).
M= Y/2 - Y₁
C-30)-(-60)
1250-1100
Y2-11
3301008
AS 0.2 KJ/mol. K
= 200 J/mol-K
1350
=
b. Calculate the AH value of the solid line labeled (a).
AG AH-TAS
= 0.2
-30 KJ/mol = AH - 1250 K x (-0.2 KJ/mol. K)
DH=280 KJ/mol
C.
At what temperature range is the reaction in the graph spontaneous?
The reaction is spontaneous when AG <O which we can
Sop in the graph in the temperature rango - 850 K to 14001.
d. Calculate the AS for the surroundings for the reaction at 1000 K.
Expert Solution
Step 1

Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning