Traces of heavy metals, such as Cu* and Pb*", in water bodies can be separated through fractional precipitation. A research work investigates the use of NaOH(a) in separating these two ions in a 1.00 L of water sampte that contains 12.70 mg Cu“" (MM = 63.546 g/mol) and 39.90 mg Pb' (MM = 399.32 g/mol). Given: Hydroxide of 2.20 x 10 Ph 1.20 x 10 5 A. Write the balanced chemical equation and the corresponding K, expression for the dissolution of the hydroxide salt of (a) Cu²* and of (b) Pb³* in water. B. Calculate the molar solubility of the hydroxide salt of (a) Cu²" and (b) Pb³* in water. If NaOH,u9) is added gradually to the water sample, which of the two ions is more likely to precipitate first? Explain your answer. c. Calculate the initial molar concentration of each of the heavy metal ions in the water sample.
Traces of heavy metals, such as Cu* and Pb*", in water bodies can be separated through fractional precipitation. A research work investigates the use of NaOH(a) in separating these two ions in a 1.00 L of water sampte that contains 12.70 mg Cu“" (MM = 63.546 g/mol) and 39.90 mg Pb' (MM = 399.32 g/mol). Given: Hydroxide of 2.20 x 10 Ph 1.20 x 10 5 A. Write the balanced chemical equation and the corresponding K, expression for the dissolution of the hydroxide salt of (a) Cu²* and of (b) Pb³* in water. B. Calculate the molar solubility of the hydroxide salt of (a) Cu²" and (b) Pb³* in water. If NaOH,u9) is added gradually to the water sample, which of the two ions is more likely to precipitate first? Explain your answer. c. Calculate the initial molar concentration of each of the heavy metal ions in the water sample.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter11: Solutions
Section: Chapter Questions
Problem 66AP: Relative solubilities of salts in liquid ammonia can differsignificantly from those in water. Thus,...
Related questions
Question
Traces of heavy metals, such as Cu2+ and Pb2+, in water bodies can be separated through fractional
precipitation. A research work investigates the use of NaOH(aq) in separating these two ions in a 1.00 L of
water sample that contains 12.70 mg Cu2+ (MM = 63.546 g/mol) and 39.90 mg Pb2+ (MM = 399.32 g/mol)
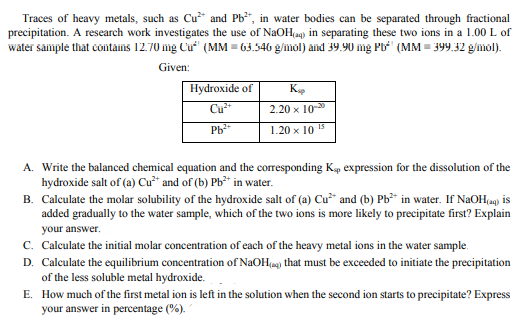
Transcribed Image Text:Traces of heavy metals, such as Cu* and Pb", in water bodies can be separated through fractional
precipitation. A research work investigates the use of NaOH(ag) in separating these two ions in a 1.00 L of
water sample that cotains 12.70 mg Cu (MM = 63.546 g/mol) and 39.90 mg Pb (MM = 399.32 g/mol).
Given:
Hydroxide of
Cu
K
2.20 х 10-3
1.20 x 10 15
A. Write the balanced chemical equation and the corresponding Kp expression for the dissolution of the
hydroxide salt of (a) Cư* and of (b) Pb** in water.
B. Calculate the molar solubility of the hydroxide salt of (a) Cu* and (b) Pb in water. If NaOH(a) is
added gradually to the water sample, which of the two ions is more likely to precipitate first? Explain
your answer.
c. Calculate the initial molar concentration of each of the heavy metal ions in the water sample.
D. Calculate the equilibrium concentration of NaOH(a) that must be exceeded to initiate the precipitation
of the less soluble metal hydroxide.
E. How much of the first metal ion is left in the solution when the second ion starts to precipitate? Express
your answer in percentage (%).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
These are continuation of the questions from above.

Transcribed Image Text:D. Calculate the equilibrium concentration of NaOHa) that must be exceeded to initiate the precipitation
of the less soluble metal hydroxide.
E. How much of the first metal ion is left in the solution when the second ion starts to precipitate? Express
your answer in percentage (%).
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning