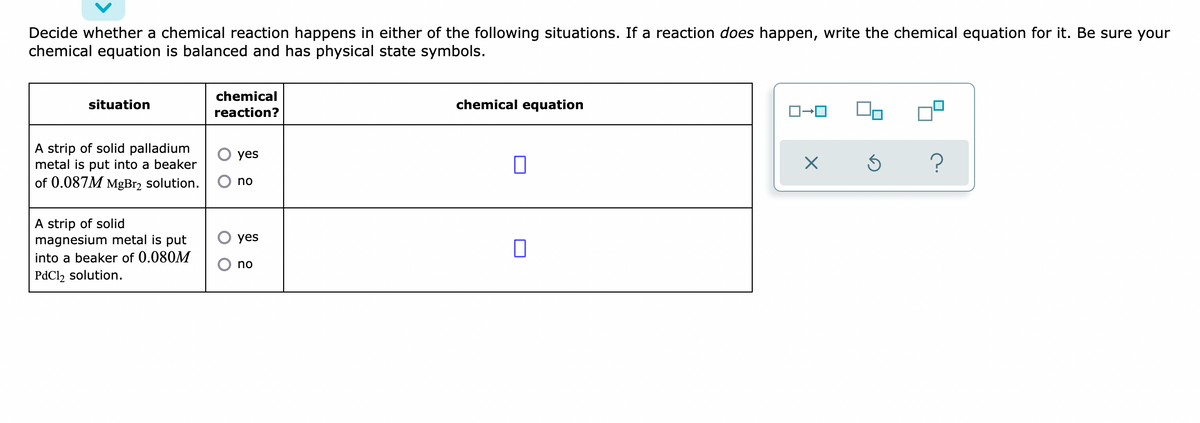
Decide whether a chemical reaction happens in either of the following situations. If a reaction does happen, write the chemical equation for it. Be sure your chemical equation is balanced and has physical state symbols. situation chemical reaction? chemical equation O yes A strip of solid palladium metal is put into a beaker of 0.087M MgBr₂ solution. ? O no O yes A strip of solid magnesium metal is put into a beaker of 0.080M PdCl₂ solution. O no ローロ X
Science behind corrosion-test
Corrosion is defined as an activity that transforms refined metals into more chemically stable forms such as oxide, hydroxide, carbonate, or sulfide. It refers to the slow decomposition of things (typically metals); thanks to chemical and/or electrochemical reactions with their surroundings. Corrosion engineering is the science of preventing and controlling corrosion.
Corrosion
Corrosion is defined as an activity that transforms refined metals into more chemically stable forms such as oxide, hydroxide, carbonate, or sulfide. It refers to the slow decomposition of things (typically metals); thanks to chemical and/or electrochemical reactions with their surroundings. Corrosion engineering is the science of preventing and controlling corrosion.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images









