Deduce the IR and Raman active carbonyl vibrations for both the cis and trans isomers of L2M(CO)4 and Fe(CO)4Cl has IR bands at 2167, 2126 and 1082 cm in CHCI3 solution. How would you interpret this spectrum and identify the isomer?
Deduce the IR and Raman active carbonyl vibrations for both the cis and trans isomers of L2M(CO)4 and Fe(CO)4Cl has IR bands at 2167, 2126 and 1082 cm in CHCI3 solution. How would you interpret this spectrum and identify the isomer?
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter14: Rotational And Vibrational Spectroscopy
Section: Chapter Questions
Problem 14.85E
Related questions
Question
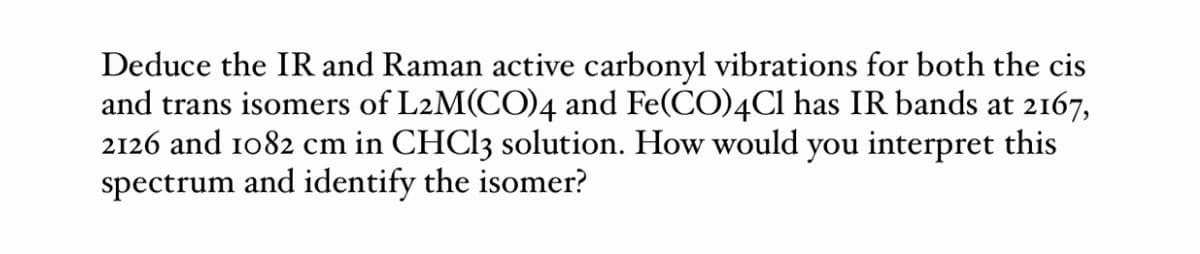
Transcribed Image Text:Deduce the IR and Raman active carbonyl vibrations for both the cis
and trans isomers of L2M(CO)4 and Fe(CO)4CI has IR bands at 2167,
2126 and 1082 cm in CHCI3 solution. How would you interpret this
spectrum and identify the isomer?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 7 steps with 7 images

Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning