Define surface tension in terms of force and show that the work done per unit area in changing the area of a liquid surface under isothermal conditions is equivalent to the definition of surface tension in terms of force
A)Define surface tension in terms of force and show that the work done per unit area in changing the area of a liquid surface under isothermal conditions is equivalent to the definition of surface tension in terms of force
Surface Tension:
Surface tension is the attractive force exerted upon the surface molecules of a liquid by the molecules beneath that tends
to draw the surface molecules into the bulk of the liquid and makes the liquid assume the shape having the least surface area.
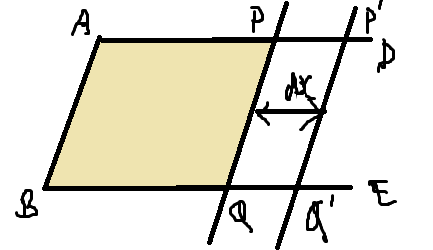
Surface tension of liquid defined as the work done per unit area is increasing the surface area of the liquid under isothermal conditions.
Mathematically, Surface tension of liquid (T)=
where W is the work done to increase the surface area by
Step by step
Solved in 3 steps with 1 images









