Dehydrohalogenation of vinyl halides is essentially an E2 process. A stercochemical study revealed that (Z)-2-chloro-2-butendioic acid reacted 50 times faster than its stereoisomer. ÇOH 1. Na+ NH2 HO,C-C=C-CO,H 2. H,0 For the reaction below: CH,CH, 1. Na NH2 2. H,0*
Dehydrohalogenation of vinyl halides is essentially an E2 process. A stercochemical study revealed that (Z)-2-chloro-2-butendioic acid reacted 50 times faster than its stereoisomer. ÇOH 1. Na+ NH2 HO,C-C=C-CO,H 2. H,0 For the reaction below: CH,CH, 1. Na NH2 2. H,0*
Chapter20: Carboxylic Acids And Nitriles
Section20.SE: Something Extra
Problem 25MP: Acid-catalyzed hydrolysis of a nitrile to give a carboxylic acid occurs by initial protonation of...
Related questions
Question
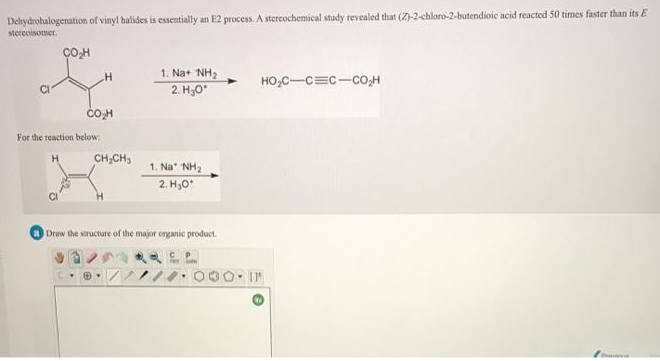
Transcribed Image Text:Dehydrohalogenation of vinyl halides is essentially an E2 process. A stercochemical study tevealed that (Z)-2-chloro-2-butendioic acid reacted 50 times faster than its E
stereoisomer.
1. Na+ NH2
HO,C-C=c-co;H
2. H,0
COH
For the reaction below
CH,CH,
1. Na NH2
2. H,0
Draw the structure of the major organic product.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning