One equivalent of 1,3-butadiene reacts with the quinone below to produce a single product. Why is there only one product, when there are several possible dienophiles in the quinone? * CH3 One of the double bonds is more reactive because it has the methyl group, an electron donating substituent. One of the double bonds is more reactive because it does not have the methyl group, an electron donating substituent The methyl group is a good leaving group, which eventually bubbles out of the solution as methane gas. Therefore, the quinone transforms into a symmetrical quinone before it reacts with the diene. Two equivalents of the unsymmetrical quinone react together to form a symmetrical quinone containing methyl groups on all four carbons.
One equivalent of 1,3-butadiene reacts with the quinone below to produce a single product. Why is there only one product, when there are several possible dienophiles in the quinone? * CH3 One of the double bonds is more reactive because it has the methyl group, an electron donating substituent. One of the double bonds is more reactive because it does not have the methyl group, an electron donating substituent The methyl group is a good leaving group, which eventually bubbles out of the solution as methane gas. Therefore, the quinone transforms into a symmetrical quinone before it reacts with the diene. Two equivalents of the unsymmetrical quinone react together to form a symmetrical quinone containing methyl groups on all four carbons.
Chapter14: Conjugated Compounds And Ultraviolet Spectroscopy
Section14.SE: Something Extra
Problem 41AP: Although the Diels–Alder reaction generally occurs between an electronrich diene and an...
Related questions
Question
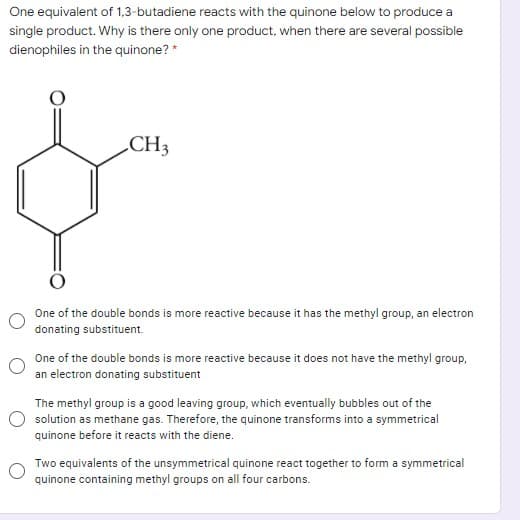
Transcribed Image Text:One equivalent of 1,3-butadiene reacts with the quinone below to produce a
single product. Why is there only one product, when there are several possible
dienophiles in the quinone? *
CH3
One of the double bonds is more reactive because it has the methyl group, an electron
donating substituent.
One of the double bonds is more reactive because it does not have the methyl group,
an electron donating substituent
The methyl group is a good leaving group, which eventually bubbles out of the
solution as methane gas. Therefore, the quinone transforms into a symmetrical
quinone before it reacts with the diene.
Two equivalents of the unsymmetrical quinone react together to form a symmetrical
quinone containing methyl groups on all four carbons.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning