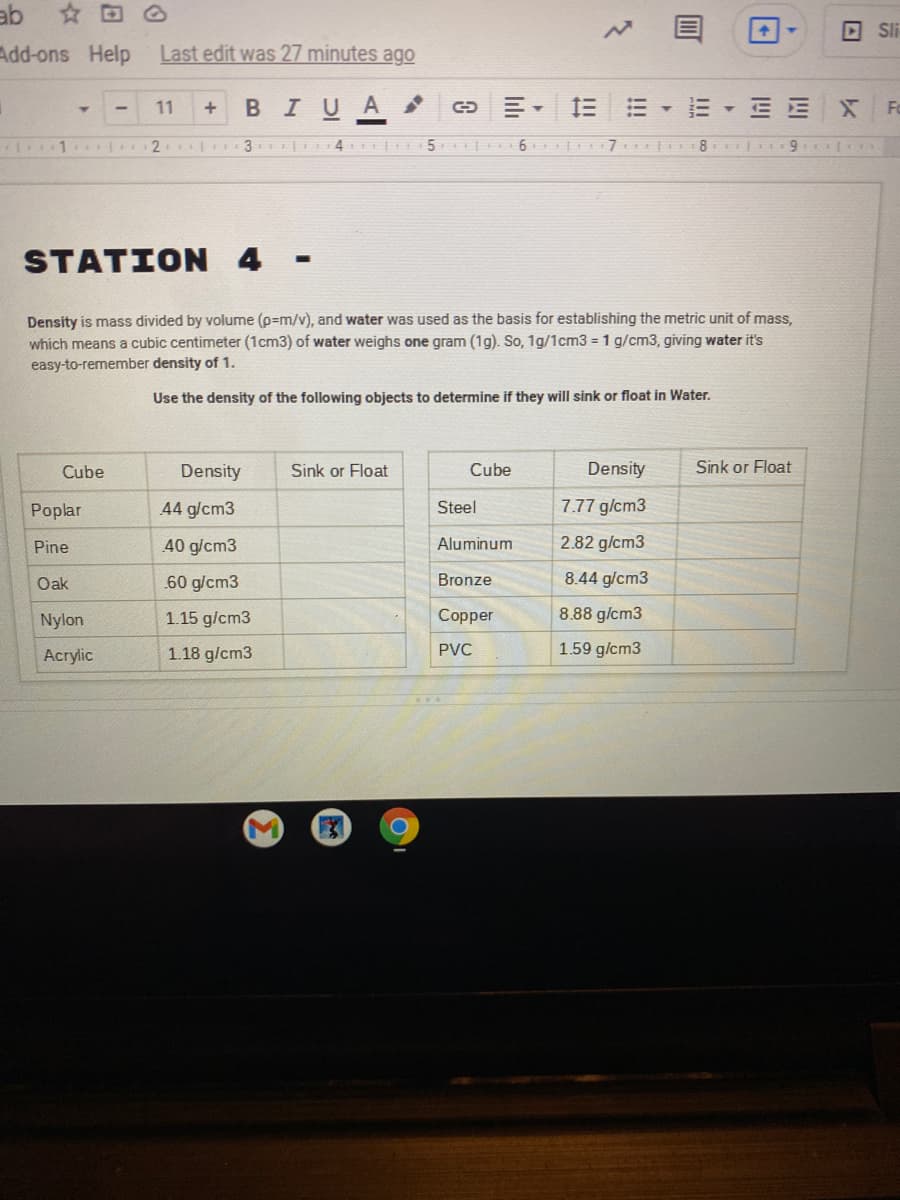
Density is mass divided by volume (p=m/v), and water was used as the basis for establishing the metric unit of mass, which means a cubic centimeter (1cm3) of water weighs one gram (1g). So, 1g/1cm3 = 1 g/cm3, giving water it's easy-to-remember density of 1. Use the density of the following objects to determine if they will sink or float in Water. Cube Density Sink or Float Cube Density Sink or Float Poplar 44 g/cm3 Steel 7.77 g/cm3 Pine 40 g/cm3 Aluminum 2.82 g/cm3 60 g/cm3 8.44 g/cm3 Oak Bronze Nylon 1.15 g/cm3 Copper 8.88 g/cm3 Acrylic 1.18 g/cm3 PVC 1.59 g/cm3
Density is mass divided by volume (p=m/v), and water was used as the basis for establishing the metric unit of mass, which means a cubic centimeter (1cm3) of water weighs one gram (1g). So, 1g/1cm3 = 1 g/cm3, giving water it's easy-to-remember density of 1. Use the density of the following objects to determine if they will sink or float in Water. Cube Density Sink or Float Cube Density Sink or Float Poplar 44 g/cm3 Steel 7.77 g/cm3 Pine 40 g/cm3 Aluminum 2.82 g/cm3 60 g/cm3 8.44 g/cm3 Oak Bronze Nylon 1.15 g/cm3 Copper 8.88 g/cm3 Acrylic 1.18 g/cm3 PVC 1.59 g/cm3
Principles of Physics: A Calculus-Based Text
5th Edition
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Raymond A. Serway, John W. Jewett
Chapter1: Introduction And Vectors
Section: Chapter Questions
Problem 7OQ: One student uses a meterstick to measure the thickness of a textbook and obtains 4.3 cm 0.1 cm....
Related questions
Question
Transcribed Image Text:ab
☆回 G
Sli
Add-ons Help
Last edit was 27 minutes ago
11
BIUA
X Fo
1
2.
6. 7 nI8.E 9
STATION
4
Density is mass divided by volume (p=m/v), and water was used as the basis for establishing the metric unit of mass,
which means a cubic centimeter (1cm3) of water weighs one gram (1g). So, 1g/1cm3 = 1 g/cm3, giving water it's
easy-to-remember density of 1.
Use the density of the following objects to determine if they will sink or float in Water.
Cube
Density
Sink or Float
Cube
Density
Sink or Float
Poplar
44 g/cm3
Steel
7.77 g/cm3
40 g/cm3
Aluminum
2.82 g/cm3
Pine
Oak
60 g/cm3
Bronze
8.44 g/cm3
Nylon
1.15 g/cm3
Copper
8.88 g/cm3
Acrylic
1.18 g/cm3
PVC
1.59 g/cm3
III
!!!
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

University Physics Volume 1
Physics
ISBN:
9781938168277
Author:
William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:
OpenStax - Rice University

Astronomy
Physics
ISBN:
9781938168284
Author:
Andrew Fraknoi; David Morrison; Sidney C. Wolff
Publisher:
OpenStax

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

University Physics Volume 1
Physics
ISBN:
9781938168277
Author:
William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:
OpenStax - Rice University

Astronomy
Physics
ISBN:
9781938168284
Author:
Andrew Fraknoi; David Morrison; Sidney C. Wolff
Publisher:
OpenStax

An Introduction to Physical Science
Physics
ISBN:
9781305079137
Author:
James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:
Cengage Learning

Glencoe Physics: Principles and Problems, Student…
Physics
ISBN:
9780078807213
Author:
Paul W. Zitzewitz
Publisher:
Glencoe/McGraw-Hill