Density of Metal Stacked Metal Coins 1. Obtain the mass of 5 coins of the same denomination. Note if you are using pennies, nickels, dimes, or quarters. 24.84 2. Measure the diameter of the stack of the coins in cm. Use this measurement to determine the radius of the coin stack. 3. Measure the height of the stacked coins in cm. 4. Using the formulas in the data collection page, determine the volume of the coins. 5. Using the mass and the volume of the coins, determine the density of the coins. 6. Using the accepted value for the metal coin density, determine the percent error. 1.6cm (Nickels) 1. Diameter of the cylinder of coins 2. Radius of the cylinder 0.8cm 3. Calculated area of base of cylinder (area = nr²) 4. Height of the cylinder 5. Calculated volume of the cylinder (volume = area base x height) 6. Mass of stacked coins 7. 8. Percent error to 3 sf Calculated density of stacked coins 0.6cm 24.84
Density of Metal Stacked Metal Coins 1. Obtain the mass of 5 coins of the same denomination. Note if you are using pennies, nickels, dimes, or quarters. 24.84 2. Measure the diameter of the stack of the coins in cm. Use this measurement to determine the radius of the coin stack. 3. Measure the height of the stacked coins in cm. 4. Using the formulas in the data collection page, determine the volume of the coins. 5. Using the mass and the volume of the coins, determine the density of the coins. 6. Using the accepted value for the metal coin density, determine the percent error. 1.6cm (Nickels) 1. Diameter of the cylinder of coins 2. Radius of the cylinder 0.8cm 3. Calculated area of base of cylinder (area = nr²) 4. Height of the cylinder 5. Calculated volume of the cylinder (volume = area base x height) 6. Mass of stacked coins 7. 8. Percent error to 3 sf Calculated density of stacked coins 0.6cm 24.84
Chapter2: Basic Statistical Analysis With Excel
Section: Chapter Questions
Problem 12P
Related questions
Question
Please help with #5
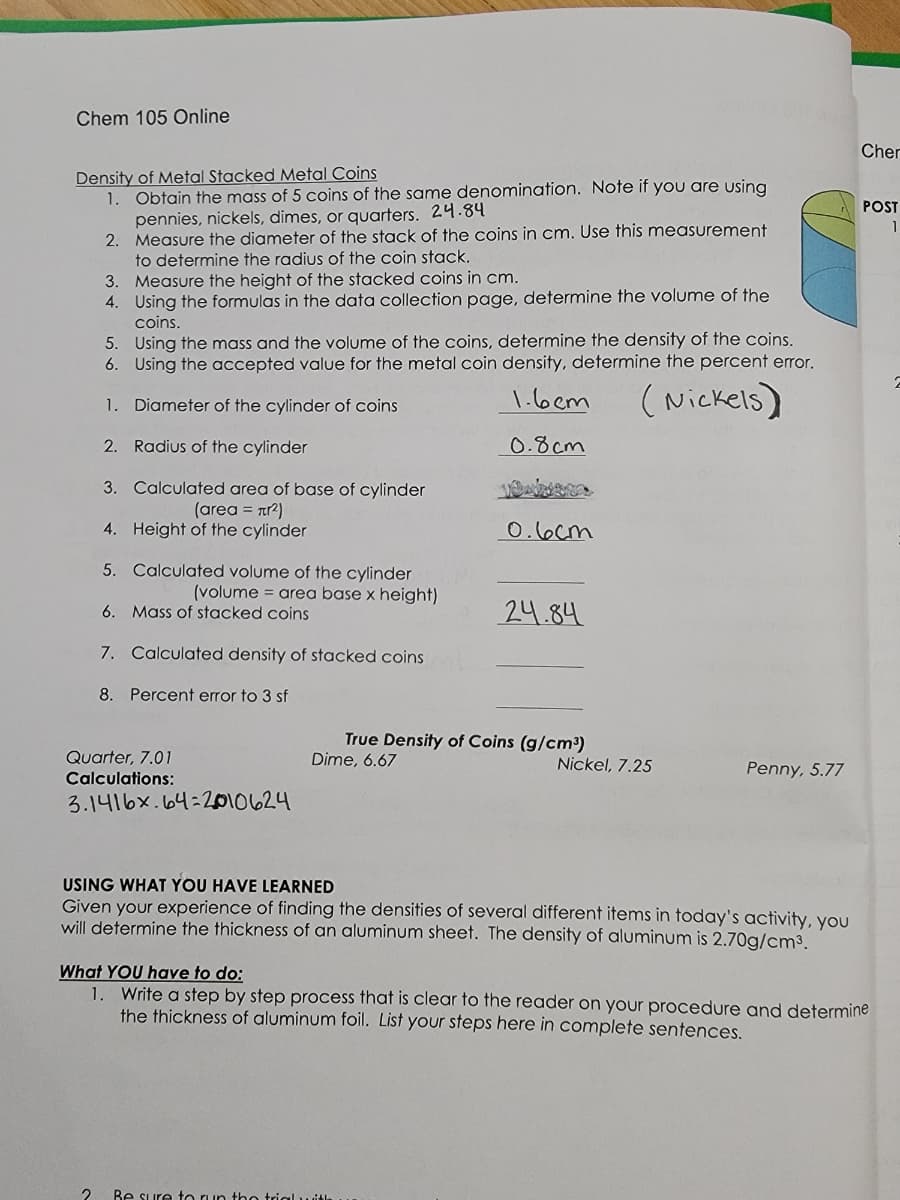
Transcribed Image Text:Chem 105 Online
Density of Metal Stacked Metal Coins
1.
Obtain the mass of 5 coins of the same denomination. Note if you are using
pennies, nickels, dimes, or quarters. 24.84
2.
Measure the diameter of the stack of the coins in cm. Use this measurement
to determine the radius of the coin stack.
3. Measure the height of the stacked coins in cm.
4. Using the formulas in the data collection page, determine the volume of the
coins.
5. Using the mass and the volume of the coins, determine the density of the coins.
6. Using the accepted value for the metal coin density, determine the percent error.
1.6cm
(Nickels)
1.
Diameter of the cylinder of coins
2.
Radius of the cylinder
0.8cm
3. Calculated area of base of cylinder
(area = л²)
4. Height of the cylinder
5.
Calculated volume of the cylinder
(volume = area base x height)
6. Mass of stacked coins
7. Calculated density of stacked coins
8. Percent error to 3 sf
Quarter, 7.01
Calculations:
2
3.1416x64:2010624
0.6cm
24.84
True Density of Coins (g/cm³)
Dime, 6.67
Be sure to run the trial with
Nickel, 7.25
USING WHAT YOU HAVE LEARNED
Given your experience of finding the densities of several different items in today's activity, you
will determine the thickness of an aluminum sheet. The density of aluminum is 2.70g/cm³.
Penny, 5.77
Cher
What YOU have to do:
1. Write a step by step process that is clear to the reader on your procedure and determine
the thickness of aluminum foil. List your steps here in complete sentences.
POST
1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you







Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co