Part D: Applying VSEPR Theory For each of the Lewis structures shown below, predict the Electron Geometry, Molecular Geometry and Bond Angle. Lastly, using the same format as shown in the last column of Table 1, draw a sketch (using wedges and dashes to show 3D if needed) of the Molecular Geometry. Lewis Total Number of Name of Name of Bond Sketch of Molecular Structure Number of Nonbonding Pairs Electron Molecular Angle Geometry Substituents Geometry Geometry S=C=$ c=ö: :Cl: H:N:H 一一
Part D: Applying VSEPR Theory For each of the Lewis structures shown below, predict the Electron Geometry, Molecular Geometry and Bond Angle. Lastly, using the same format as shown in the last column of Table 1, draw a sketch (using wedges and dashes to show 3D if needed) of the Molecular Geometry. Lewis Total Number of Name of Name of Bond Sketch of Molecular Structure Number of Nonbonding Pairs Electron Molecular Angle Geometry Substituents Geometry Geometry S=C=$ c=ö: :Cl: H:N:H 一一
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter10: Molecular Structure And Bonding Theories
Section: Chapter Questions
Problem 10.43QE: For each of the following molecules, complete the Lewis structure and use the VSEPR model to...
Related questions
Question
I am slightly confused on how to do this. I think I figured it out but I just want to compare data and take notes on what I have done incorrectly.
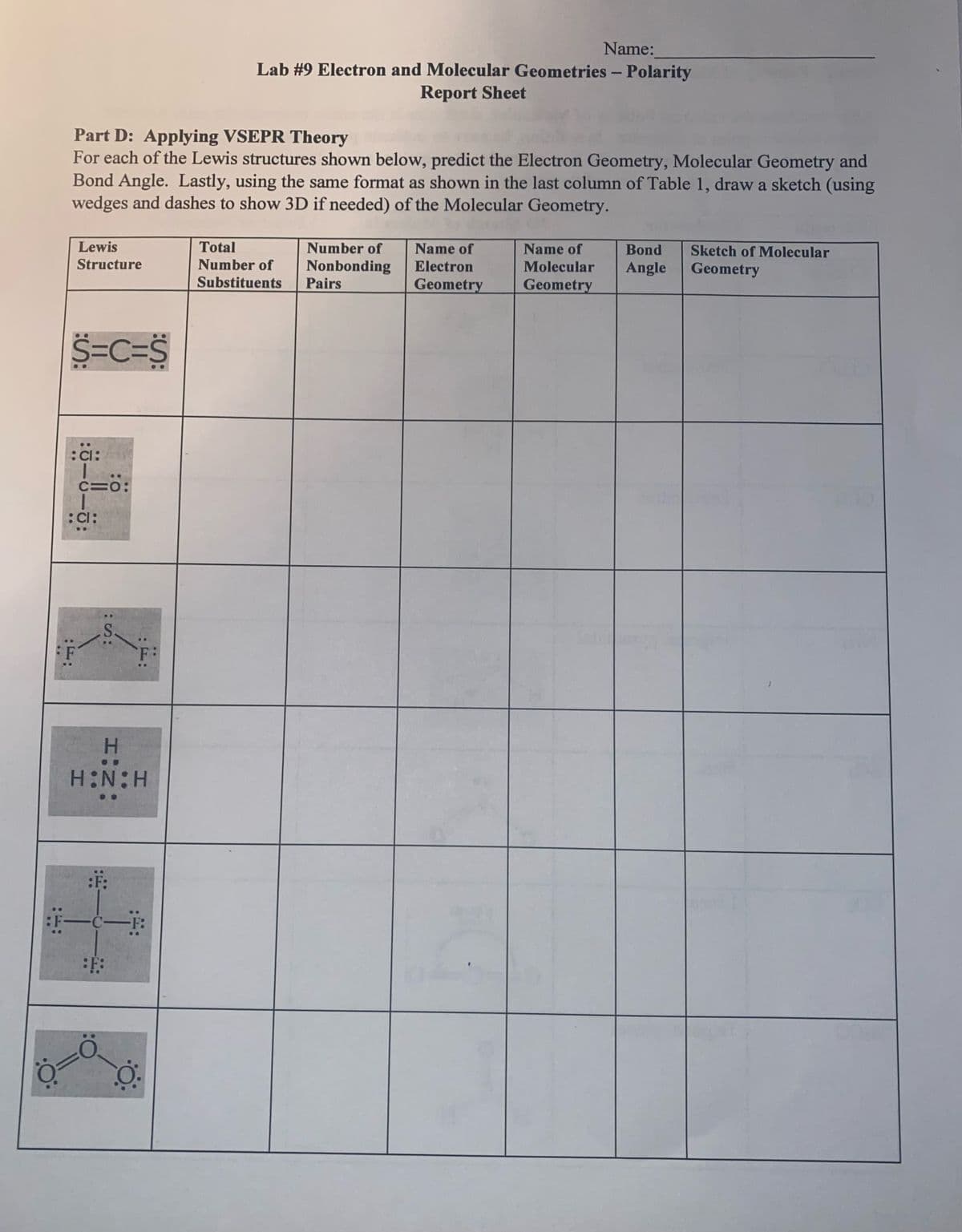
Transcribed Image Text:Name:
Lab #9 Electron and Molecular Geometries - Polarity
Report Sheet
Part D: Applying VSEPR Theory
For each of the Lewis structures shown below, predict the Electron Geometry, Molecular Geometry and
Bond Angle. Lastly, using the same format as shown in the last column of Table 1, draw a sketch (using
wedges and dashes to show 3D if needed) of the Molecular Geometry.
Lewis
Structure
Total
Number of
Name of
Name of
Bond
Sketch of Molecular
Number of
Nonbonding
Pairs
Electron
Molecular
Angle
Geometry
Substituents
Geometry
Geometry
S=C=S
:Cl:
C=0:
:Cl:
..
F
H.
HN:H
一一
-F:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 6 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning