Density of Unknown Metal 23 Temperature of water: C Density of water: g/cm Trial 1 Trial 2 Trial 3 Trial 4 Mass of empty flask +stopper 29.391 g 29.30l0 8 29,3g29. 391g Mass of stoppered flask +water SI.002 g0l. 582 gol,500 8 l.570 g (to fill the flask) Mass of water 31. 045 32,214 g 31.179 g32.189 (to fill the flask) Internal volume of flask 21,74 cm3 32.29 cm3 32.20 cm32. 25 cm (see Appendix H) Mass of metal 29.0398 21,0448 21,01 821.041g Mass of stoppered flask + metal + 71. 191 g17.4918 11.419 17.940 8 water Mass of water (surrounding the metal) Volume of water (surrounding the cm3 cm cm cm metal) Volume of metal ст3 Cm 3 cm cm3 DENSITY OF METAL g/cm3 g/cm3 g/cm3 g/cm3 ab bb
Density of Unknown Metal 23 Temperature of water: C Density of water: g/cm Trial 1 Trial 2 Trial 3 Trial 4 Mass of empty flask +stopper 29.391 g 29.30l0 8 29,3g29. 391g Mass of stoppered flask +water SI.002 g0l. 582 gol,500 8 l.570 g (to fill the flask) Mass of water 31. 045 32,214 g 31.179 g32.189 (to fill the flask) Internal volume of flask 21,74 cm3 32.29 cm3 32.20 cm32. 25 cm (see Appendix H) Mass of metal 29.0398 21,0448 21,01 821.041g Mass of stoppered flask + metal + 71. 191 g17.4918 11.419 17.940 8 water Mass of water (surrounding the metal) Volume of water (surrounding the cm3 cm cm cm metal) Volume of metal ст3 Cm 3 cm cm3 DENSITY OF METAL g/cm3 g/cm3 g/cm3 g/cm3 ab bb
Chapter1: Matter, Measurements, And Calculations
Section: Chapter Questions
Problem 1.127E
Related questions
Question
How do I find the mass of water and volume of water that are empty. I know the formulas but which numbers do I use?
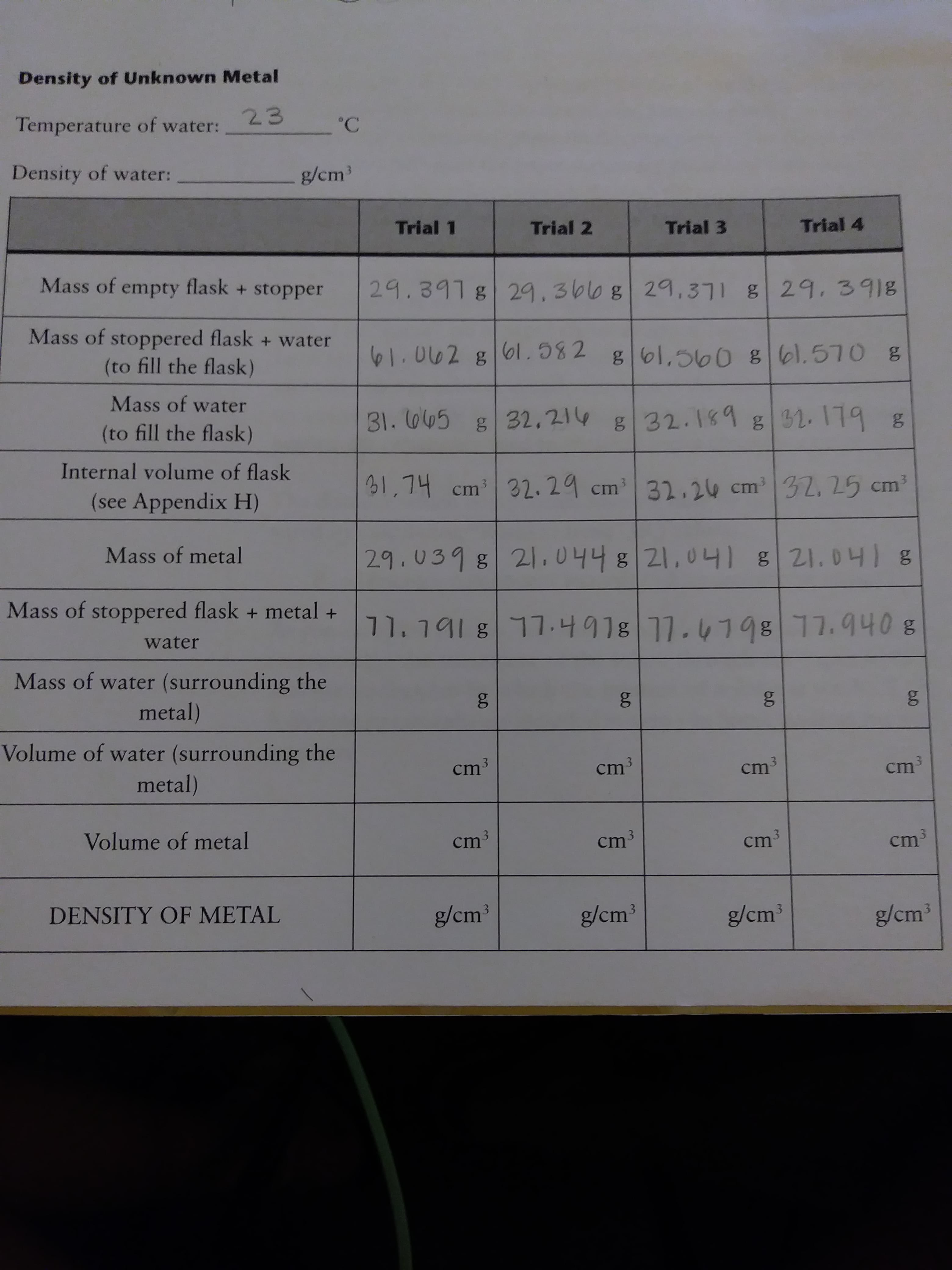
Transcribed Image Text:Density of Unknown Metal
23
Temperature of water:
C
Density of water:
g/cm
Trial 1
Trial 2
Trial 3
Trial 4
Mass of empty flask +stopper
29.391 g 29.30l0 8 29,3g29. 391g
Mass of stoppered flask +water
SI.002 g0l. 582
gol,500 8 l.570 g
(to fill the flask)
Mass of water
31. 045
32,214
g 31.179
g32.189
(to fill the flask)
Internal volume of flask
21,74 cm3
32.29 cm3 32.20 cm32. 25 cm
(see Appendix H)
Mass of metal
29.0398 21,0448 21,01 821.041g
Mass of stoppered flask + metal +
71. 191 g17.4918 11.419 17.940 8
water
Mass of water (surrounding the
metal)
Volume of water (surrounding the
cm3
cm
cm
cm
metal)
Volume of metal
ст3
Cm 3
cm
cm3
DENSITY OF METAL
g/cm3
g/cm3
g/cm3
g/cm3
ab
bb
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 6 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning